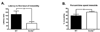Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6) - PubMed (original) (raw)
Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6)
B C McKinney et al. Genes Brain Behav. 2008 Aug.
Abstract
The Scn8a gene encodes the alpha-subunit of Na(v)1.6, a neuronal voltage-gated sodium channel. Mice homozygous for mutations in the Scn8a gene exhibit motor impairments. Recently, we described a human family with a heterozygous protein truncation mutation in SCN8A. Rather than motor impairment, neuropsychological abnormalities were more common, suggesting a role for Scn8a in a more diverse range of behaviors. Here, we characterize mice heterozygous for a null mutation of Scn8a (Scn8a(+/-)mice) in a number of behavioral paradigms. We show that Scn8a(+/-)mice exhibit greater conditioned freezing in the Pavlovian fear conditioning paradigm but no apparent abnormalities in other learning and memory paradigms including the Morris water maze and conditioned taste avoidance paradigm. Furthermore, we find that Scn8a(+/-)mice exhibit more pronounced avoidance of well-lit, open environments as well as more stress-induced coping behavior. Together, these data suggest that Scn8a plays a critical role in emotional behavior in mice. Although the behavioral phenotype observed in the Scn8a(+/-)mice only partially models the abnormalities in the human family, we anticipate that the Scn8a(+/-)mice will serve as a valuable tool for understanding the biological basis of emotion and the human diseases in which abnormal emotional behavior is a primary component.
Figures
Figure 1
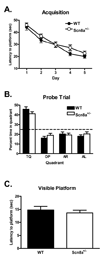
Among mice remaining after those exhibiting excessive floating were eliminated from analysis, there was no difference in performance between _Scn8a+/−and_WT mice in the Morris water maze. (A) The time to reach the hidden platform during training was not significantly different Scn8a+/− mice (N = 28) when compared WT mice (N = 26). (B) A 60 second probe trial completed 24 hours after the last training trial (day 6) reveals that both Scn8a+/− mice and WT mice spend a significant amount of time during the trial searching in the quadrant where the platform was previously located (TQ; training quadrant) but there was no significant difference between the genotypes. The dashed line (25%) represents random performance. (AR, AL, OP abbreviated for
A
djacent
R
ight,
A
djacent
L
eft &
O
pposite, respectively). (C) Average latency to platform for Scn8a+/− mice during the visible-platform version of the Morris water maze was not significantly different when compared with WT mice. All data are presented as mean ± S.E.M.
Figure 2
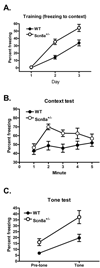
Scn8a+/− mice exhibit greater freezing to training context and tone than WT mice. (A) As training progressed, both Scn8a+/− mice (N = 15) and WT mice (N = 13) exhibited increases in freezing to training context with Scn8a+/− mice freezing significantly more than WT mice. (B) Scn8a+/− mice exhibited greater freezing to context upon 5 minute exposure to training context than WT mice. (C) Scn8a+/− mice exhibited greater generalization of fear to the reconfigured context and greater freezing to tone than WT mice. All data are presented as mean ± S.E.M.
Figure 3
Scn8a+/− mice extinguish contextually-conditioned fear as well as WT mice. (A) Both Scn8a+/− mice (N = 31) and WT mice (N = 24) exhibited increases in freezing as training progressed with Scn8a+/− mice consistently freezing more than WT mice in the intervals following and between shocks. (B) Scn8a+/− mice (N = 21) exhibited more freezing than WT mice (N = 16) across a one-hour exposure to the conditioning chambers, but there was no genotype-training interaction. (C) Twenty-four hours after extinction training, mice were re-exposed to the conditioning chambers. Scn8a+/− mice froze significantly more than WT mice and retention control mice froze more than mice in the extinction group, but there was no interaction between genotype or group. All data are presented as mean ± S.E.M. (*) p < 0.05 two-way ANOVA; training group factor.
Figure 4
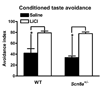
Scn8a+/− mice (N = 7) and WT (N = 5) mice learn to avoid saccharin following a saccharin-lithium chloride pairing equally well. All data are presented as mean ± S.E.M. (*) p < 0.05; two-way ANOVA treatment group factor.
Figure 5
Scn8a+/− mice exhibit more pronounced avoidance of the center zone of the open field. (A) Total distance traveled by Scn8a+/− mice (N = 12) was not significantly different when compared to WT mice (N = 9). (B) Scn8a+/− mice, however, on average traveled less distance in the center of the open field when compared to WT mice. All data are presented as mean ± S.E.M. (*) p < 0.05; unpaired t-test.
Figure 6
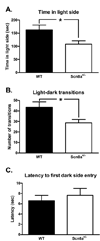
Scn8a+/− mice exhibit more pronounced avoidance of the light side of the light-dark box than WT mice. (A) Scn8a+/− mice (N = 12) spent less time exploring the aversive light side of the light-dark box than WT mice (N = 9). (B) Scn8a+/− mice made significantly fewer transitions between sides of the light-dark box than WT mice. (C) Upon initial placement in the light side of the light-dark box, latency to first entry into the dark side did not differ between Scn8a+/− and WT mice. All data are presented as mean ± S.E.M. (*) p < 0.05; unpaired t-test.
Figure 7
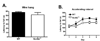
Scn8a+/− mice exhibit increased immobility in the forced swim test. (A) Latency to first bout of immobility was significantly shorter in Scn8a+/− mice (N = 12) than WT mice (N = 9). (B) Scn8a+/− mice spent significantly more total time immobile than WT mice. All data are presented as mean ± S.E.M. (*) p < 0.05; unpaired t-test.
Figure 8
Motor performance is unimpaired in Scn8a+/− mice. (A) Neuromuscular strength as assessed by latency to fall from an inverted wire mesh did not differ between Scn8a+/− mice (N = 12) and their WT littermates (N = 9). (B) Motor coordination and balance as assessed by latency to fall from an accelerating rotarod did not differ between Scn8a+/− mice and their WT littermates. All data are presented as mean ± S.E.M.
Similar articles
- Dysfunction of the Scn8a voltage-gated sodium channel alters sleep architecture, reduces diurnal corticosterone levels, and enhances spatial memory.
Papale LA, Paul KN, Sawyer NT, Manns JR, Tufik S, Escayg A. Papale LA, et al. J Biol Chem. 2010 May 28;285(22):16553-61. doi: 10.1074/jbc.M109.090084. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20353942 Free PMC article. - The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy.
Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. Martin MS, et al. Hum Mol Genet. 2007 Dec 1;16(23):2892-9. doi: 10.1093/hmg/ddm248. Epub 2007 Sep 19. Hum Mol Genet. 2007. PMID: 17881658 - Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice.
Papale LA, Beyer B, Jones JM, Sharkey LM, Tufik S, Epstein M, Letts VA, Meisler MH, Frankel WN, Escayg A. Papale LA, et al. Hum Mol Genet. 2009 May 1;18(9):1633-41. doi: 10.1093/hmg/ddp081. Epub 2009 Mar 2. Hum Mol Genet. 2009. PMID: 19254928 Free PMC article. - Sodium channels and neurological disease: insights from Scn8a mutations in the mouse.
Meisler MH, Kearney J, Escayg A, MacDonald BT, Sprunger LK. Meisler MH, et al. Neuroscientist. 2001 Apr;7(2):136-45. doi: 10.1177/107385840100700208. Neuroscientist. 2001. PMID: 11496924 Review. - Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions.
Meisler MH, Plummer NW, Burgess DL, Buchner DA, Sprunger LK. Meisler MH, et al. Genetica. 2004 Sep;122(1):37-45. doi: 10.1007/s10709-004-1441-9. Genetica. 2004. PMID: 15619959 Review.
Cited by
- Prefrontal PV interneurons facilitate attention and are linked to attentional dysfunction in a mouse model of absence epilepsy.
Ferguson B, Glick C, Huguenard JR. Ferguson B, et al. Elife. 2023 Apr 4;12:e78349. doi: 10.7554/eLife.78349. Elife. 2023. PMID: 37014118 Free PMC article. - Social Deficits and Cerebellar Degeneration in Purkinje Cell Scn8a Knockout Mice.
Yang X, Yin H, Wang X, Sun Y, Bian X, Zhang G, Li A, Cao A, Li B, Ebrahimi-Fakhari D, Yang Z, Meisler MH, Liu Q. Yang X, et al. Front Mol Neurosci. 2022 Apr 26;15:822129. doi: 10.3389/fnmol.2022.822129. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35557557 Free PMC article. - Sodium channelopathies in neurodevelopmental disorders.
Meisler MH, Hill SF, Yu W. Meisler MH, et al. Nat Rev Neurosci. 2021 Mar;22(3):152-166. doi: 10.1038/s41583-020-00418-4. Epub 2021 Feb 2. Nat Rev Neurosci. 2021. PMID: 33531663 Free PMC article. Review. - Scn8a Antisense Oligonucleotide Is Protective in Mouse Models of SCN8A Encephalopathy and Dravet Syndrome.
Lenk GM, Jafar-Nejad P, Hill SF, Huffman LD, Smolen CE, Wagnon JL, Petit H, Yu W, Ziobro J, Bhatia K, Parent J, Giger RJ, Rigo F, Meisler MH. Lenk GM, et al. Ann Neurol. 2020 Mar;87(3):339-346. doi: 10.1002/ana.25676. Epub 2020 Feb 6. Ann Neurol. 2020. PMID: 31943325 Free PMC article. - Environmental variables that ameliorate extinction learning deficits in the 129S1/SvlmJ mouse strain.
Cazares VA, Rodriguez G, Parent R, Ouillette L, Glanowska KM, Moore SJ, Murphy GG. Cazares VA, et al. Genes Brain Behav. 2019 Sep;18(7):e12575. doi: 10.1111/gbb.12575. Epub 2019 May 7. Genes Brain Behav. 2019. PMID: 30973205 Free PMC article.
References
- Aguilar R, Gil L, Flint J, Gray JA, Dawson GR, Driscoll P, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Tobena A. Learned fear, emotional reactivity and fear of heights: a factor analytic map from a large F2 intercross of Roman rat strains. Brain Res Bull. 2002;57:17–26. - PubMed
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. - PubMed
- Broadhurst PL. Determinants of Emotionality in the Rat .1. Situational Factors. Br J Psychol. 1957;48:1–12. - PubMed
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant 'motor endplate disease'. Nat Genet. 1995;10:461–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034509-10S1/NS/NINDS NIH HHS/United States
- 5T32GM008322/GM/NIGMS NIH HHS/United States
- T32 GM008322/GM/NIGMS NIH HHS/United States
- R01 NS034509/NS/NINDS NIH HHS/United States
- T32GM007544/GM/NIGMS NIH HHS/United States
- NS34509/NS/NINDS NIH HHS/United States
- T32 GM007544/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases