Polarized immune responses differentially regulate cancer development - PubMed (original) (raw)
Review
Polarized immune responses differentially regulate cancer development
Magnus Johansson et al. Immunol Rev. 2008 Apr.
Abstract
Tumor-associated immune responses assert varied responses toward developing neoplasms that can either act to eradicate malignant cells via engagement of potent cytotoxic programs or alternatively enhance tumor growth through release of multifunctional pro-tumor mediators. Seemingly paradoxical, these disparate activities reflect a continuum of polarization (or activation) states possible for distinct leukocyte subsets that demonstrate tissue, organ, and tumor selectivity. Herein, we review clinical and experimental studies investigating cellular and molecular mechanisms utilized by neoplastic tissues to alternatively polarize immune responses that favor either pro- or anti-tumor immunity.
Figures
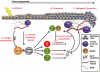
Fig. 1. Polarization of immune responses during tumor development
Epithelial cancers develop through expansion of cells harboring mutations in key regulatory genes, i.e. initiated cells (A). Microenvironmental effectors, such as inflammation, promote (B) malignant conversion (C) of initiated cells. When an inflammatory response is triggered during initiation or promotion, antigen-presenting cells (APCs), such as dendritic cells and macrophages, take up tumor-associated antigens and present them (D) to naïve T helper (Th) cells. Depending on the context and the inflammatory microenvironment in which antigen presentation occurs, Th cells can become alternatively polarized. The process of immune polarization integrates a large number of microenvironmental signals (E) into one general outcome. Three discrete and mutually exclusive Th cell lineages, Th1, Th2, and Th17, have been defined. Th1 polarized immune responses (F) are associated with cytotoxic T lymphocytes (CTL)-mediated killing of tumor cells and favor cancer regression. CTL-mediated tumor cell cytotoxicity can be inhibited through recruitment and/or conversion of natural or adaptive regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) (G). Th2 and Th17 polarized immune responses both have been shown to promote tumor progression (H) by establishing chronic inflammation, e.g. through cytokine production and induction of humoral responses. Chronic inflammation can promote tumor progression in multiple ways, such as stimulation of tumor cell proliferation and dedifferentiation, activation of angiogenesis, and recruitment of Tregs and MDSCs, as well as through remodeling of extracellular matrix (ECM) and tissue basement membranes.
Similar articles
- Multiple roles for CD4+ T cells in anti-tumor immune responses.
Kennedy R, Celis E. Kennedy R, et al. Immunol Rev. 2008 Apr;222:129-44. doi: 10.1111/j.1600-065X.2008.00616.x. Immunol Rev. 2008. PMID: 18363998 Review. - The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance.
Allavena P, Sica A, Garlanda C, Mantovani A. Allavena P, et al. Immunol Rev. 2008 Apr;222:155-61. doi: 10.1111/j.1600-065X.2008.00607.x. Immunol Rev. 2008. PMID: 18364000 Review. - The inflammatory tumor microenvironment and its impact on cancer development.
de Visser KE, Coussens LM. de Visser KE, et al. Contrib Microbiol. 2006;13:118-137. doi: 10.1159/000092969. Contrib Microbiol. 2006. PMID: 16627962 Review. - Tumor-associated macrophages: from mechanisms to therapy.
Noy R, Pollard JW. Noy R, et al. Immunity. 2014 Jul 17;41(1):49-61. doi: 10.1016/j.immuni.2014.06.010. Immunity. 2014. PMID: 25035953 Free PMC article. Review. - Tissue-resident cytotoxic innate lymphoid cells in tumor immunosurveillance.
Stamatiades EG, Li MO. Stamatiades EG, et al. Semin Immunol. 2019 Feb;41:101269. doi: 10.1016/j.smim.2019.03.001. Epub 2019 Mar 21. Semin Immunol. 2019. PMID: 30904283 Free PMC article. Review.
Cited by
- Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma.
Kinoshita T, Ishii G, Hiraoka N, Hirayama S, Yamauchi C, Aokage K, Hishida T, Yoshida J, Nagai K, Ochiai A. Kinoshita T, et al. Cancer Sci. 2013 Apr;104(4):409-15. doi: 10.1111/cas.12099. Epub 2013 Feb 19. Cancer Sci. 2013. PMID: 23305175 Free PMC article. - Pre-clinical studies of Notch signaling inhibitor RO4929097 in inflammatory breast cancer cells.
Debeb BG, Cohen EN, Boley K, Freiter EM, Li L, Robertson FM, Reuben JM, Cristofanilli M, Buchholz TA, Woodward WA. Debeb BG, et al. Breast Cancer Res Treat. 2012 Jul;134(2):495-510. doi: 10.1007/s10549-012-2075-8. Epub 2012 May 1. Breast Cancer Res Treat. 2012. PMID: 22547109 Free PMC article. - Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment.
Demehri S, Turkoz A, Kopan R. Demehri S, et al. Cancer Cell. 2009 Jul 7;16(1):55-66. doi: 10.1016/j.ccr.2009.05.016. Cancer Cell. 2009. PMID: 19573812 Free PMC article. - Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family.
Shepard HM, Brdlik CM, Schreiber H. Shepard HM, et al. J Clin Invest. 2008 Nov;118(11):3574-81. doi: 10.1172/JCI36049. J Clin Invest. 2008. PMID: 18982164 Free PMC article. Review. - T cells in health and disease.
Sun L, Su Y, Jiao A, Wang X, Zhang B. Sun L, et al. Signal Transduct Target Ther. 2023 Jun 19;8(1):235. doi: 10.1038/s41392-023-01471-y. Signal Transduct Target Ther. 2023. PMID: 37332039 Free PMC article. Review.
References
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. - PubMed
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol Mech Dis. 2006;1:119–150. - PubMed
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA098075/CA/NCI NIH HHS/United States
- R01 CA094168/CA/NCI NIH HHS/United States
- R01 CA094168-05/CA/NCI NIH HHS/United States
- CA72006/CA/NCI NIH HHS/United States
- P01 CA072006/CA/NCI NIH HHS/United States
- CA098075/CA/NCI NIH HHS/United States
- R01 CA098075-05/CA/NCI NIH HHS/United States
- P01 CA072006-06A29002/CA/NCI NIH HHS/United States
- U54 RR020843-045942/RR/NCRR NIH HHS/United States
- CA94168/CA/NCI NIH HHS/United States
- U54 RR020843/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources