Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line - PubMed (original) (raw)
Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line
Hyuntae Yoo et al. J Biol Chem. 2008.
Abstract
We previously reported that glutamine was a major source of carbon for de novo fatty acid synthesis in a brown adipocyte cell line. The pathway for fatty acid synthesis from glutamine may follow either of two distinct pathways after it enters the citric acid cycle. The glutaminolysis pathway follows the citric acid cycle, whereas the reductive carboxylation pathway travels in reverse of the citric acid cycle from alpha-ketoglutarate to citrate. To quantify fluxes in these pathways we incubated brown adipocyte cells in [U-(13)C]glutamine or [5-(13)C]glutamine and analyzed the mass isotopomer distribution of key metabolites using models that fit the isotopomer distribution to fluxes. We also investigated inhibitors of NADP-dependent isocitrate dehydrogenase and mitochondrial citrate export. The results indicated that one third of glutamine entering the citric acid cycle travels to citrate via reductive carboxylation while the remainder is oxidized through succinate. The reductive carboxylation flux accounted for 90% of all flux of glutamine to lipid. The inhibitor studies were compatible with reductive carboxylation flux through mitochondrial isocitrate dehydrogenase. Total cell citrate and alpha-ketoglutarate were near isotopic equilibrium as expected if rapid cycling exists between these compounds involving the mitochondrial membrane NAD/NADP transhydrogenase. Taken together, these studies demonstrate a new role for glutamine as a lipogenic precursor and propose an alternative to the glutaminolysis pathway where flux of glutamine to lipogenic acetyl-CoA occurs via reductive carboxylation. These findings were enabled by a new modeling tool and software implementation (Metran) for global flux estimation.
Figures
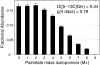
FIGURE 1.
Mass isotopomer distribution of palmitate isolated from WT brown adipocytes under incubation in medium containing 4 mm [5-13C]glutamine from day 2 to day 6 (mean ± S. E.;n = 3). Contribution of [5-13C]glutamine to palmitate synthesis (D value) and fractional new synthesis of palmitate (g value) determined by ISA.
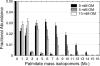
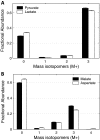
FIGURE 2.
Effect of oxalomalate on palmitate synthesis from [U-13C]glutamine. Shown is the mass isotopomer distribution of palmitate from WT brown adipocytes on day 4 under 6 h of incubation in medium containing 4 m
m
[U-13C]glutamine and 0, 5, or 10 m
m
oxalomalate (Error bars, mean ± S.E.; n = 3).
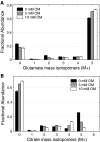
FIGURE 3.
Effect of increasing concentration of oxalomalate (A) and 2-methylisocitrate (B) on D(Gln) and g(6 h) values of palmitate synthesis from [U-13C]glutamine and from [U-13C]glucose (C). Asterisk (*) indicates significant difference compared with the control condition without inhibitor (p < 0.005). Error bars, mean ± S.E.
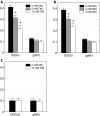
FIGURE 4.
Effect of increasing concentration of oxalomalate on labeling patterns of key intermediates of CAC. Mass isotopomer distributions of glutamate (A) and citrate (B) from WT brown adipocytes on day 4 under 6 h of incubation in medium containing 4 m
m
[U-13C]glutamine and 0, 5, or 10 m
m
oxalomalate. Data were corrected for natural isotope enrichments. Error bars, mean ± S.E.
FIGURE 5.
Flux from [U-13C]glucose to key intermediates of glycolysis and CAC. Mass isotopomer distributions of pyruvate and lactate (A) and malate and aspartate (B) from WT brown adipocytes on day 4 under 6 h of incubation in medium containing 25 m
m
[U-13C]glucose and 4 m
m
unlabeled glutamine. Data were corrected for natural isotope enrichments. Error bars, mean ± S.E.
Similar articles
- Fatty acid labeling from glutamine in hypoxia can be explained by isotope exchange without net reductive isocitrate dehydrogenase (IDH) flux.
Fan J, Kamphorst JJ, Rabinowitz JD, Shlomi T. Fan J, et al. J Biol Chem. 2013 Oct 25;288(43):31363-9. doi: 10.1074/jbc.M113.502740. Epub 2013 Sep 12. J Biol Chem. 2013. PMID: 24030823 Free PMC article. - Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability.
Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Wise DR, et al. Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19611-6. doi: 10.1073/pnas.1117773108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106302 Free PMC article. - Glutamine metabolism in AS-30D hepatoma cells. Evidence for its conversion into lipids via reductive carboxylation.
Holleran AL, Briscoe DA, Fiskum G, Kelleher JK. Holleran AL, et al. Mol Cell Biochem. 1995 Nov 22;152(2):95-101. doi: 10.1007/BF01076071. Mol Cell Biochem. 1995. PMID: 8751155 - Metabolic and mind shifts: from glucose to glutamine and acetate addictions in cancer.
Corbet C, Feron O. Corbet C, et al. Curr Opin Clin Nutr Metab Care. 2015 Jul;18(4):346-53. doi: 10.1097/MCO.0000000000000178. Curr Opin Clin Nutr Metab Care. 2015. PMID: 26001655 Review. - Carboxylation and anaplerosis in neurons and glia.
Hassel B. Hassel B. Mol Neurobiol. 2000 Aug-Dec;22(1-3):21-40. doi: 10.1385/MN:22:1-3:021. Mol Neurobiol. 2000. PMID: 11414279 Review.
Cited by
- Evidence for transketolase-like TKTL1 flux in CHO cells based on parallel labeling experiments and (13)C-metabolic flux analysis.
Ahn WS, Crown SB, Antoniewicz MR. Ahn WS, et al. Metab Eng. 2016 Sep;37:72-78. doi: 10.1016/j.ymben.2016.05.005. Epub 2016 May 10. Metab Eng. 2016. PMID: 27174718 Free PMC article. - The Role of Mitochondrial NADPH-Dependent Isocitrate Dehydrogenase in Cancer Cells.
Smolková K, Ježek P. Smolková K, et al. Int J Cell Biol. 2012;2012:273947. doi: 10.1155/2012/273947. Epub 2012 May 20. Int J Cell Biol. 2012. PMID: 22675360 Free PMC article. - Dynamic metabolic reprogramming in dendritic cells: An early response to influenza infection that is essential for effector function.
Rezinciuc S, Bezavada L, Bahadoran A, Duan S, Wang R, Lopez-Ferrer D, Finkelstein D, McGargill MA, Green DR, Pasa-Tolic L, Smallwood HS. Rezinciuc S, et al. PLoS Pathog. 2020 Oct 26;16(10):e1008957. doi: 10.1371/journal.ppat.1008957. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33104753 Free PMC article. - Lipokine 5-PAHSA Is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for De Novo Lipogenesis in Mice.
Paluchova V, Oseeva M, Brezinova M, Cajka T, Bardova K, Adamcova K, Zacek P, Brejchova K, Balas L, Chodounska H, Kudova E, Schreiber R, Zechner R, Durand T, Rossmeisl M, Abumrad NA, Kopecky J, Kuda O. Paluchova V, et al. Diabetes. 2020 Mar;69(3):300-312. doi: 10.2337/db19-0494. Epub 2019 Dec 5. Diabetes. 2020. PMID: 31806624 Free PMC article. - Studying metabolic flux adaptations in cancer through integrated experimental-computational approaches.
Lagziel S, Lee WD, Shlomi T. Lagziel S, et al. BMC Biol. 2019 Jul 4;17(1):51. doi: 10.1186/s12915-019-0669-x. BMC Biol. 2019. PMID: 31272436 Free PMC article. Review.
References
- Newsholme, E. A., Crabtree, B., and Ardawi, M. S. (1985) Biosci. Rep. 5 393–400 - PubMed
- Yoo, H., Stephanopoulos, G., and Kelleher, J. K. (2004) J. Lipid Res. 45 1324–1332 - PubMed
- Mazurek, S., and Eigenbrodt, E. (2003) Anticancer Res. 23 1149–1154 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources