Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme - PubMed (original) (raw)
Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme
Jayaraj Rajagopal et al. Development. 2008 May.
Abstract
The effects of Wnt7b on lung development were examined using a conditional Wnt7b-null mouse. Wnt7b-null lungs are markedly hypoplastic, yet display largely normal patterning and cell differentiation. In contrast to findings in prior hypomorphic Wnt7b models, we find decreased replication of both developing epithelium and mesenchyme, without abnormalities of vascular smooth muscle development. We further demonstrate that Wnt7b signals to neighboring cells to activate both autocrine and paracrine canonical Wnt signaling cascades. In contrast to results from hypomorphic models, we show that Wnt7b modulates several important signaling pathways in the lung. Together, these cascades result in the coordinated proliferation of adjacent epithelial and mesenchymal cells to stimulate organ growth with few alterations in differentiation and patterning.
Figures
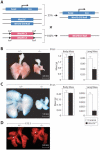
Fig. 1. Deletion of mouse Wnt7b results in hypoplastic lungs
(A) Strategy to remove Wnt7b from only the embryo proper. (B) Mutant lungs are 50% smaller than wild-type at E18.5. The overall mass of the embryo is unchanged. (C) Mutant lungs are 75% smaller than wild-type at E14.5. The overall mass of the embryo is unchanged. (D) Control and mutant lungs at E12.5. E-cadherin staining shows grossly normal lobulation in mutant lungs. However, the right medial lobe bronchus (*) of the Wnt7b mutant lung emanates from the cephalic lobe bronchus.
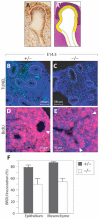
Fig. 2. Cell fate specification, timing of differentiation and tissue architecture are unchanged in _Wnt7b_-null lungs
(A-D′) E14.5 immunohistochemistry of heterozygous (A-D) and Wnt7b mutant (A′-D′) mouse lungs. Blood vessels appear normal in the mutant, as marked by PECAM (A,A′), as does airway smooth muscle, marked by smooth muscle myosin (B,B′). Proximal Sox2 (C,C′) and distal Sox9 (D,D′) expression are unchanged in mutants. Dotted lines encircle the epithelium. (E-I′) At E18.5, immunohistochemistry of heterozygous (E-I) and Wnt7b mutant (E′-I′) lungs demonstrates that blood vessels (E,E′), airway smooth muscle cells (F,F′), Clara cells as marked by CC10 (G,G′), and type 2 cells as marked by surfactant protein C (H,H′) are unchanged in mutant lungs. Type 1 cells marked by caveolin 1 (I,I′) are decreased in number. (J) Quantification of cell types in E18.5 lungs demonstrates that the proportions of Clara, ciliated and mucous cells are unchanged in the trachea and airways of Wnt7b mutants. In the distal saccules, the proportions of cells positive for SP-C or PECAM are also unchanged. Error bars represent 1 s.d. (K) The number of Sox9-positive cells per lung bud tip at E14.5 is unchanged in Wnt7b mutants.
Fig. 3. Deletion of Wnt7b results in proportionate decreases in replication of epithelial and mesenchymal tip cells
(A,A′) Schematic depiction (A′) of BrdU staining (brown) of E14.5 lung bud tip (A). Replication is highest in tip epithelium (A′, dark yellow) and tip mesenchyme (A′, dark purple) as compared with proximal epithelium (A′, light yellow) and mesenchyme (A′, light purple). (B,C TUNEL staining of E14.5 wild-type (B) and Wnt7b mutant (C) mouse lungs shows no increase in apoptosis in the mutant. Dotted lines encircle epithelium. (D,E) BrdU staining of E14.5 wild-type (D) and mutant (E) lungs demonstrates decreased replication in mesenchymal and epithelial cells of the mutant. Arrowheads point to the tips. Dotted lines encircle epithelium. (F) A comparison of the percentage of BrdU-labeled cells in wild-type (gray) and mutant (white) tips demonstrates proportionate drops in epithelial and mesenchymal replication (P<0.001).
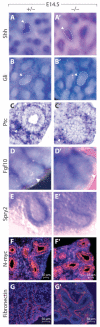
Fig. 4. Preserved expression of many factors affecting growth in Wnt7b mutant lungs
(A-E′) E14.5 ISH of Wnt7b mutant (A′-E′) and control (A-E) mouse lungs. Expression of Shh (A,A′), Gli1 (B,B′), Ptch1 (C,C′) and Spry2 (E,E′) are unchanged in the mutant. The epithelial tip is encircled. Fgf10 staining (D,D′) is largely preserved in mutant lungs; there is a variable decrease in the most-distal expression of Fgf10 in mutant lungs (arrowhead). (F-G′) E14.5 immunohistochemistry for N-myc (F,F′) and fibronectin expression (G,G′) showing that these are unchanged in the mutant. ISH signal (purple) is indicated by the arrowheads.
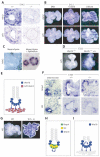
Fig. 5. Mechanism of Wnt7b action in mouse lung development
(A) ISH showing loss of canonical Wnt targets in mesenchyme and endoderm of Wnt7b mutant lungs. Dotted lines encircle epithelium. Arrowheads point to periendodermal mesenchyme. (B) E12.5 whole-mount ISH of Axin2 and Lef1 in cultured wild-type lungs. Wnt signaling is decreased with the addition of Dkk1 (left) and enhanced by lithium (right) when compared with control cultures (middle). In lithium-treated lungs, mesenchymal staining of the canonical targets has expanded. (C) Axin2 expression in mesenchyme cultured alone and in mesenchyme recombined with epithelium, showing that mesenchymal Axin2 expression requires endoderm induction. (D) Lithium rescues Lef1 expression in E12.5 mutant lungs when compared with Wnt7b mutant lungs cultured without lithium. (E) Epithelial Wnt7b activates a canonical pathway in neighboring mesenchyme. (F) Bmp4 and Id2 expression are coincident on serial sections of E14.5 wild-type lung (left). Expression of epithelial Bmp4 and Id2 are decreased in mutant lungs (middle and right). (G) Lithium induces ectopic Id2 expression in cultured E12.5 wild-type lungs. (H) Wnt7b induces Bmp4 and Id2 production in endoderm tip cells. (I) Wnt7b coordinately activates autocrine and paracrine signaling cascades to increase cell replication.
Similar articles
- Wnt7b is required for epithelial progenitor growth and operates during epithelial-to-mesenchymal signaling in pancreatic development.
Afelik S, Pool B, Schmerr M, Penton C, Jensen J. Afelik S, et al. Dev Biol. 2015 Mar 15;399(2):204-17. doi: 10.1016/j.ydbio.2014.12.031. Epub 2015 Jan 7. Dev Biol. 2015. PMID: 25576928 - Wnt7b regulates mesenchymal proliferation and vascular development in the lung.
Shu W, Jiang YQ, Lu MM, Morrisey EE. Shu W, et al. Development. 2002 Oct;129(20):4831-42. doi: 10.1242/dev.129.20.4831. Development. 2002. PMID: 12361974 - Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5.
Wang Z, Shu W, Lu MM, Morrisey EE. Wang Z, et al. Mol Cell Biol. 2005 Jun;25(12):5022-30. doi: 10.1128/MCB.25.12.5022-5030.2005. Mol Cell Biol. 2005. PMID: 15923619 Free PMC article. - Epithelial-mesenchymal crosstalk in Wolffian duct and fetal testis cord development.
Archambeault DR, Tomaszewski J, Joseph A, Hinton BT, Yao HH. Archambeault DR, et al. Genesis. 2009 Jan;47(1):40-8. doi: 10.1002/dvg.20453. Genesis. 2009. PMID: 18979542 Free PMC article. Review. - FGF10: A multifunctional mesenchymal-epithelial signaling growth factor in development, health, and disease.
Itoh N. Itoh N. Cytokine Growth Factor Rev. 2016 Apr;28:63-9. doi: 10.1016/j.cytogfr.2015.10.001. Epub 2015 Oct 31. Cytokine Growth Factor Rev. 2016. PMID: 26559461 Review.
Cited by
- Expression of fibroblast growth factor 9 in normal human lung and idiopathic pulmonary fibrosis.
Coffey E, Newman DR, Sannes PL. Coffey E, et al. J Histochem Cytochem. 2013 Sep;61(9):671-9. doi: 10.1369/0022155413497366. Epub 2013 Jun 24. J Histochem Cytochem. 2013. PMID: 23797050 Free PMC article. - How to Use Online Tools to Generate New Hypotheses for Mammary Gland Biology Research: A Case Study for Wnt7b.
van de Grift YBC, Heijmans N, van Amerongen R. van de Grift YBC, et al. J Mammary Gland Biol Neoplasia. 2020 Dec;25(4):319-335. doi: 10.1007/s10911-020-09474-z. Epub 2021 Feb 24. J Mammary Gland Biol Neoplasia. 2020. PMID: 33625717 Free PMC article. - LRP5 in age-related changes in vascular and alveolar morphogenesis in the lung.
Mammoto A, Muyleart M, Mammoto T. Mammoto A, et al. Aging (Albany NY). 2019 Jan 5;11(1):89-103. doi: 10.18632/aging.101722. Aging (Albany NY). 2019. PMID: 30612120 Free PMC article. - The role of pleiotrophin and beta-catenin in fetal lung development.
Weng T, Liu L. Weng T, et al. Respir Res. 2010 Jun 18;11(1):80. doi: 10.1186/1465-9921-11-80. Respir Res. 2010. PMID: 20565841 Free PMC article. Review. - Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development.
Volckaert T, De Langhe SP. Volckaert T, et al. Dev Dyn. 2015 Mar;244(3):342-66. doi: 10.1002/dvdy.24234. Epub 2014 Dec 29. Dev Dyn. 2015. PMID: 25470458 Free PMC article. Review.
References
- Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997a;124:53–63. - PubMed
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997b;124:4867–4878. - PubMed
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995;172:126–138. - PubMed
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. - PubMed
- Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397:617–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials