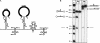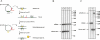Maturation of the 5S rRNA 5' end is catalyzed in vitro by the endonuclease tRNase Z in the archaeon H. volcanii - PubMed (original) (raw)
Maturation of the 5S rRNA 5' end is catalyzed in vitro by the endonuclease tRNase Z in the archaeon H. volcanii
Annette Hölzle et al. RNA. 2008 May.
Abstract
Ribosomal RNA molecules are synthesized as precursors that have to undergo several processing steps to generate the functional rRNA. The 5S rRNA in the archaeon Haloferax volcanii is transcribed as part of a multicistronic transcript containing both large rRNAs and one or two tRNAs. Release of the 5S rRNA from the precursor requires two endonucleolytic cleavages by enzymes as yet not identified. Here we report the first identification of an archaeal 5S rRNA processing endonuclease. The enzyme tRNase Z, which was initially identified as tRNA processing enzyme, generates not only tRNA 3' ends but also mature 5S rRNA 5' ends in vitro. Interestingly, the sequence upstream of the 5S rRNA can be folded into a mini-tRNA, which might explain the processing of this RNA by tRNase Z. The endonuclease is active only at low salt concentrations in vitro, which is in contrast to the 2-4 M KCl concentration present inside the cell in vivo. Electron microscopy studies show that there are no compartments inside the Haloferax cell that could provide lower salt environments. Processing of the 5S rRNA 5' end is not restricted to the haloarchaeal tRNase Z since tRNase Z enzymes from a thermophilic archaeon, a lower and a higher eukaryote, are as well able to cleave the tRNA-like structure 5' of the 5S rRNA. Knock out of the tRNase Z gene in Haloferax volcanii is lethal, showing that the protein is essential for the cell.
Figures
FIGURE 1.
(A) Structure of the multicistronic rRNA transcript. The H. volcanii genome contains two copies of the rRNA operon, which differ in the 3′ part. Both operons contain the genes for 16S rRNA, tRNAAla, 23S rRNA, and the 5S rRNA, but only one operon encodes in addition the distal tRNACys. The primary transcript is processed by several endonucleolytic cuts (indicated by black arrows). The tRNAs are removed by RNase P and tRNase Z, and the 16S rRNA and the 23S rRNA are removed from the primary transcript by the splicing endonuclease (SE), which cleaves at the bulge-helix-bulge motifs. Both large rRNAs still contain additional 5′ and 3′ sequences, which have to be removed by as yet unidentified endonucleases or exonucleases. The 5S rRNA is also excised as pre-5S rRNA by as yet unidentified endonucleases. Potentially remaining nucleotides have to be removed by exonucleases. (B) A protein extract from H. volcanii processes the 5S rRNA precursor. Two different 5S precursors were incubated with a soluble protein extract from H. volcanii. The longer precursor contains the 5S rRNA and 5′ as well as 3′ additional sequences (lanes: long). The short precursor contains only the 5S rRNA and the 5′ extension (lanes: short). (Lanes p) Incubation with protein extract; (lanes c) incubation without proteins. (Lane m) DNA size marker, sizes are given at the left. Precursor and products from the long precursor are shown schematically at the right; precursor and products from the short precursor are shown at the left.
FIGURE 2.
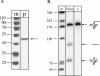
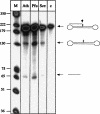
Expression of the H. volcanii tRNase Z gene. The gene for the Haloferax tRNase Z was cloned into expression vector pET29a (Novagen), expressed in E. coli, and purified using the S-tag (Novagen). Purification yields a pure recombinant tRNase Z as shown by the silver-stained SDS PAGE. (Lane m) Protein size marker; sizes are given in kDa at the left. (Lane p) Recombinant tRNase Z, indicated by the arrow.
FIGURE 3.
The H. volcanii tRNase Z catalyzes the 5S rRNA 5′ end processing reaction. (A) Uniformly labeled 5S rRNA substrate. The long 5S precursor was incubated with the H. volcanii tRNase Z. (Lane c) Incubation without proteins; (lane p) incubation with tRNase Z. (Lane m) DNA size marker; sizes are shown at the left. Precursor and products are shown schematically at the right. (B) 3′ end labeled 5S rRNA substrate. The short 5S precursor was labeled at the 3′ end and incubated with the recombinant H. volcanii tRNase Z. (Lane c) Incubation without proteins; (lane p) incubation with tRNase Z. (Lane m) DNA size marker; sizes are shown at the right. Precursor and products are shown schematically at the left.
FIGURE 4.
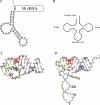
Structure of the 5S rRNA leader sequence. (A) Using the program RNAfold of the Vienna RNA package (
http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi
), a potential two-dimensional structure of the 5′ leader sequence was calculated. (B) The canonical tRNA secondary structure is shown. Conserved nucleotides are indicated. (C) A 3D model structure was generated based on the similarity of the 5S RNA leader sequence and predicted secondary structure to the tRNA acceptor stem, T-stem, and T-loop regions (see Materials and Methods). Bases that correspond to acceptor stem, T-stem, and T-loop regions are indicated as blue, red, and green stick representation, respectively (nucleic acid backbone in gray). For clarity, only the backbone trace of the D-replacement loop is shown except for two guanine bases (atom color code) that allow for similar D-loop/T-loop contacts as seen in tRNA structures. (D) Structure of yeast tRNAPhe with the same view and color coding as in B (only the backbone trace is shown for the whole D-arm and anti-codon-arm region).
FIGURE 5.
Determination of the cleavage site. 5′ leader products were analyzed in respect to the cleavage site of tRNase Z. The majority of clones obtained showed that cleavage occurs between nucleotides −1 and +1 of the 5S rRNA precursor.
FIGURE 6.
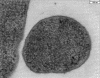
Electron microscopy of H. volcanii cells. H. volcanii cells were high-pressure frozen and freeze-substituted for electron microscopic analysis.
FIGURE 7.
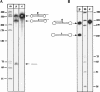
The 5S rRNA precursor is also processed by tRNase Z enzymes from another archaeon and eukaryotes. tRNase Z enzymes from Saccharomyces cerevisiae (lane Sce), Arabidopsis thaliana (tRNase ZS1; lane Ath), and Pyrococcus furiosus (lane Pfu) were incubated with the short 5S rRNA substrate. (Lane M) DNA size marker; sizes are given at the left. (Lane c) Control reaction without the addition of proteins. Precursor and products are shown schematically at the right.
FIGURE 8.
Knock out of the tRNase Z gene is lethal. The gene for tRNase Z in the Haloferax volcanii genome was replaced by the trpA gene by the pop-in/pop-out transformation procedure. (A) Schematic presentation of the knock out strategy. SalI sites important for Southern hybridizations are indicated (S). Strain H119 containing the wild-type trz gene (shown in yellow and labeled hvotrz) was transformed with the integrating plasmid pTA131-Z1/4-trpA. Successful integrants (pop-in events) were selected on medium without tryptophan and uracil. The plasmid was forced out by plating the cells on 5-FOA, which yielded a heterozygous pop-out (B). This pop-out was transformed with plasmid pTA409-zwt, which contains the wild-type trz gene. After several cycles of cultivation, a homozygous strain was obtained (C). (B) The deletion of the chromosomal trz gene was investigated by Southern analysis. As a probe, the upstream sequence of the trz gene was used (probe Z1/2). Replacement of the trz gene by the trpA gene results in a 864-bp SalI fragment, whereas the wild-type gene gives rise to a 1754-bp fragment. Both fragments are visible, indicating that cells still contain wild-type copies of the gene in some of the chromosomal DNA copies present in the Haloferax cells. (Lanes a_–_d) Heterozygous pop-out strains; (lane e) wild-type strain. (C) To generate a homozygous chromosomal knock out strain, the heterozygous cells were transformed with plasmid pTA409-zwt, which contains the tRNase Z gene. Southern analysis of this strain shows that it is homozygous for the pop-out. Only the 864-bp SalI fragment of the pop-out is visible and the plasmid borne trz gene, which is 6223 bp. (Lane a) Wild-type strain; (lane b) heterozygous strain without the plasmid (as in B); (lane c) homozygous pop-out strain. A DNA size marker is given at the right in base pairs.
Similar articles
- tRNA-like elements in Haloferax volcanii.
Hölzle A, Stoll B, Schnattinger T, Schöning U, Tjaden B, Marchfelder A. Hölzle A, et al. Biochimie. 2012 Apr;94(4):940-6. doi: 10.1016/j.biochi.2011.12.002. Epub 2011 Dec 8. Biochimie. 2012. PMID: 22178322 - Two archaeal tRNase Z enzymes: similar but different.
Späth B, Schubert S, Lieberoth A, Settele F, Schütz S, Fischer S, Marchfelder A. Späth B, et al. Arch Microbiol. 2008 Sep;190(3):301-8. doi: 10.1007/s00203-008-0368-4. Epub 2008 Apr 25. Arch Microbiol. 2008. PMID: 18437358 - tRNA 3' end maturation in archaea has eukaryotic features: the RNase Z from Haloferax volcanii.
Schierling K, Rösch S, Rupprecht R, Schiffer S, Marchfelder A. Schierling K, et al. J Mol Biol. 2002 Mar 1;316(4):895-902. doi: 10.1006/jmbi.2001.5395. J Mol Biol. 2002. PMID: 11884130 - The making of tRNAs and more - RNase P and tRNase Z.
Hartmann RK, Gössringer M, Späth B, Fischer S, Marchfelder A. Hartmann RK, et al. Prog Mol Biol Transl Sci. 2009;85:319-68. doi: 10.1016/S0079-6603(08)00808-8. Prog Mol Biol Transl Sci. 2009. PMID: 19215776 Review. - Transfer RNA processing in archaea: unusual pathways and enzymes.
Heinemann IU, Söll D, Randau L. Heinemann IU, et al. FEBS Lett. 2010 Jan 21;584(2):303-9. doi: 10.1016/j.febslet.2009.10.067. FEBS Lett. 2010. PMID: 19878676 Free PMC article. Review.
Cited by
- tRNA biology charges to the front.
Phizicky EM, Hopper AK. Phizicky EM, et al. Genes Dev. 2010 Sep 1;24(17):1832-60. doi: 10.1101/gad.1956510. Genes Dev. 2010. PMID: 20810645 Free PMC article. Review. - Comprehensive analysis of the pre-ribosomal RNA maturation pathway in a methanoarchaeon exposes the conserved circularization and linearization mode in archaea.
Qi L, Li J, Jia J, Yue L, Dong X. Qi L, et al. RNA Biol. 2020 Oct;17(10):1427-1441. doi: 10.1080/15476286.2020.1771946. Epub 2020 Jun 19. RNA Biol. 2020. PMID: 32449429 Free PMC article. - Effect of changes in the flexible arm on tRNase Z processing kinetics.
Levinger L, Hopkinson A, Desetty R, Wilson C. Levinger L, et al. J Biol Chem. 2009 Jun 5;284(23):15685-91. doi: 10.1074/jbc.M900745200. Epub 2009 Apr 7. J Biol Chem. 2009. PMID: 19351879 Free PMC article. - Identification of essential and non-essential single-stranded DNA-binding proteins in a model archaeal organism.
Skowyra A, MacNeill SA. Skowyra A, et al. Nucleic Acids Res. 2012 Feb;40(3):1077-90. doi: 10.1093/nar/gkr838. Epub 2011 Oct 5. Nucleic Acids Res. 2012. PMID: 21976728 Free PMC article. - An archaeal immune system can detect multiple protospacer adjacent motifs (PAMs) to target invader DNA.
Fischer S, Maier LK, Stoll B, Brendel J, Fischer E, Pfeiffer F, Dyall-Smith M, Marchfelder A. Fischer S, et al. J Biol Chem. 2012 Sep 28;287(40):33351-63. doi: 10.1074/jbc.M112.377002. Epub 2012 Jul 5. J Biol Chem. 2012. PMID: 22767603 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources