WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression - PubMed (original) (raw)
WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression
Jie Jia et al. Mol Cell. 2008.
Abstract
The heterotetrameric GAIT complex suppresses translation of selected mRNAs in interferon-gamma-activated monocytic cells. Specificity is dictated by glutamyl-prolyl tRNA synthetase (EPRS) binding to a 3'UTR element in target mRNAs. EPRS consists of two synthetase cores joined by a linker containing three WHEP domains of unknown function. Here we show the critical role of EPRS WHEP domains in targeting and regulating GAIT complex binding to RNA. The upstream WHEP pair directs high-affinity binding to GAIT element-bearing mRNAs, while the overlapping, downstream pair binds NSAP1, which inhibits mRNA binding. Interaction of EPRS with ribosomal protein L13a and GAPDH induces a conformational switch that rescues mRNA binding and restores translational control. Total reconstitution from purified components indicates that the four GAIT proteins are necessary and sufficient for self-assembly of a functional complex. Our results establish the essentiality of WHEP domains in the noncanonical function of EPRS in regulating inflammatory gene expression.
Figures
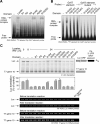
Figure 1. EPRS Linker Domain Binds the RNA GAIT Element
(A) Sequence and sub-domains of human EPRS. The N-terminus EF1Bγ-like domain and ERS domain are connected to the C-terminus PRS domain by a linker consisting of three WHEP repeats, R1, R2, and R3, separated by two spacers, S1 and S2. (B) EPRS linker domain binds the GAIT element. Major EPRS domains, i.e., ERS (including N-terminus EF1Bγ-like domain), linker, and PRS, were cloned and expressed in E. coli as recombinant His-tagged proteins, purified by Ni-affinity chromatography, and assessed by SDS-PAGE and Coomassie stain (left). Recombinant EPRS domains were incubated with 32P-labeled Cp GAIT element RNA and subjected to RNA EMSA and autoradiography (center). The interaction was confirmed by UV-crosslinking to the same RNA followed by SDS-PAGE (right). (C) SPR analysis of EPRS linker binding to the GAIT element. Biotinylated Cp GAIT element (left) and the U87C mutant (right) RNAs were immobilized on a streptavidin sensor chip (Biacore). Purified, recombinant human EPRS linker was injected at concentrations from 3 to 200 nM. After 3 min at a flow rate of 30 μl/min, the solution was replaced with buffer to permit dissociation. EPRS binding was determined by SPR and expressed as resonance units (RU). Binding KD was determined by Biaevaluation software. (D) Specific binding of EPRS linker to GAIT element RNA. Biotinylated, wild-type Cp GAIT element was immobilized on a streptavidin sensor chip. EPRS linker (100 nM) was pre-incubated for 30 min with wild-type (●) or U87C mutant (○) GAIT element RNA (12.5 to 400 nM) as competitor. Relative binding efficiency is expressed as mean ± standard error of the mean (s.e.m., n = 3 experiments).
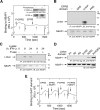
Figure 2. Upstream Pair of EPRS WHEP Domains Binds the GAIT Element
(A) Interaction between GAIT element and EPRS linker WHEP domains. N-terminus, His-tagged linker containing 1, 2, or 3 WHEP domains with appropriate spacers were expressed in E. coli and affinity-purified. Recombinant linker sub-domains were incubated with 32P-labeled Cp GAIT element RNA, and binding evaluated by EMSA. (B) Role of inter-WHEP domain spacers in binding to GAIT element. N-terminus, His-tagged linker domains containing WHEP repeat pairs separated by the correct or incorrect spacer were expressed in E. coli and affinity-purified. Recombinant sub-domains were incubated with 32P-labeled Cp GAIT element RNA (left) or inactive mutant GAIT element RNA (right), and the interaction determined by EMSA. (C) Functional analysis of binding of WHEP domains to GAIT element. U937 cells were treated with IFN-γ for 0, 8, or 24 h and cytosolic extracts prepared. Translational silencing was measured using a reporter RNA containing Luc ORF (including the first 55 nt of the Luc 3'UTR), the 29-nt Cp GAIT element, and a 30-nt poly(A) tail. T7 gene 10 RNA was co-translated as a control for specificity. Linker sub-domains (40 pmol) were used as decoys and pre-incubated with the transcripts. The protein-RNA mixtures were subjected to in vitro translation in a rabbit reticulocyte lysate in the presence of [35S] methionine and the 24-h cytosolic extract. Newly translated Luc and T7 gene 10 were resolved by SDS-PAGE electrophoresis and detected by autoradiography (top panel). Luc expression was quantitated by densitometry, normalized by T7 gene 10 expression, and expressed as mean ± s.e.m. (n = 4 experiments) (2nd panel). The linker domains that significantly blocked translational silencing are denoted with an asterisk (P<0.05, two-tailed T-test). The stability of Luc and T7 gene 10 reporter mRNAs was determined by RT-PCR before and after the in vitro translation reactions (3rd through 6th panels).
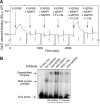
Figure 3. NSAP1 Binds Downstream Pair of EPRS WHEP Repeats and Blocks Binding to GAIT Element
(A) NSAP1 binds phospho-EPRS. C-terminal His-tagged, full-length human EPRS was expressed in rabbit reticulocyte lysate to generate phospho-EPRS (inset, top). Recombinant protein was immunoprecipitated (IP) with anti-human EPRS antibody and phosphorylation determined by immunoblot (IB) with anti-phosphoserine (P-Ser) antibody. Dephosphorylated EPRS was prepared by incubation with alkaline phosphatase-coupled agarose beads. Total expression of human EPRS was determined by IB with rabbit anti-EPRS antibody (inset, bottom). Recombinant human NSAP1 was immobilized on a Biacore CM5 chip for determination of EPRS binding by SPR. Phospho-EPRS (P-EPRS) or dephosphorylated EPRS (100 nM) were separately injected (closed arrows) for 3 min, and then replaced with buffer (open arrows) to allow dissociation. Binding was expressed as resonance units (RU). (B) NSAP1 binds EPRS in the linker domain. Recombinant ERS, linker, and PRS domains were expressed and purified as in Figure 1B. Cytosol from 8-h, IFN-γ-treated cells was added to provide NSAP1 and to induce EPRS domain phosphorylation. The lysates were subjected to immunoprecipitation (IP) with anti-NSAP1 antibody and immunoblotted (IB) with anti-His tag (top) or anti-NSAP1 (bottom) antibodies. (C) EPRS linker phosphorylation is required for binding to NSAP1. Recombinant EPRS linker containing the 3 WHEP domains was incubated with cytosol prepared from U937 cells incubated with IFN-γ for up to 24 h. Some samples were then treated with alkaline phosphatase as in (A) to dephosphorylate the linker. NSAP1 was immunoprecipitated with anti-NSAP1 antibody and the linker detected by immunoblot with anti-His tag antibody (top). The blot was stripped and reprobed with anti-NSAP1 antibody as loading control (bottom). (D) Identification of R2R3 as the minimal NSAP1-binding domain. Recombinant WHEP domains R1, R2, R3, R1R2, R2R3, and R1R2R3 were incubated with cytosol prepared from U937 cells treated with IFN-γ for 8 h. The mixtures were immunoprecipitated with anti-His tag antibody and immunoblotted with anti-NSAP1 antibody (top). Samples not subjected to immunoprecipitation were immunoblotted with anti-NSAP1 antibody (bottom). (E) NSAP1 blocks phospho-EPRS binding to GAIT element RNA. Biotinylated Cp GAIT element RNA was immobilized on a streptavidin chip. C-terminus, Full-length, His-tagged EPRS and P-EPRS (10 pmol) were pre-incubated for 30 min with a 10-fold molar excess of human NSAP1 expressed in insect cells. In four consecutive injections, binding of EPRS, EPRS plus NSAP1, P-EPRS, and P-EPRS plus NSAP1 was measured by SPR and expressed as resonance units (RU).
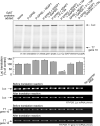
Figure 4. Reconstitution of RNA-Binding Activity of the GAIT Complex
(A) Reconstitution of GAIT complex RNA-binding activity. Biotinylated GAIT element RNA was immobilized on a streptavidin chip. Phospho-EPRS (P-EPRS) was pre-incubated with a 10-fold molar excess of NSAP1. In other samples, to the phospho-EPRS and NSAP1 mixture were added GAPDH, phosphorylated L13a, GAPDH plus phosphorylated L13a, or GAPDH plus unmodified L13a (all added at 10-fold molar excess compared to P-EPRS). Binding to immobilized Cp GAIT element RNA was determined by SPR and expressed as resonance units (RU). (B) Determination of proteins in the reconstituted GAIT element-binding complex by electrophoretic mobility supershift analysis. Phospho-EPRS, NSAP1, phospho-L13a, and GAPDH (5 pmol of each protein) were pre-incubated for 60 min at room temperature, and then added to 32P-labeled Cp GAIT element probe. The solution was subjected to EMSA in the presence of polyclonal antibody (1 μg) against each of the four GAIT complex proteins.
Figure 5. Reconstitution of Translational Silencing Function of the GAIT Complex
Phospho-EPRS was pre-incubated with NSAP1, and with other GAIT complex components as indicated (5 pmol of each protein), and in vitro translation was measured using a wheat germ extract in the presence of 35S-labeled Met and the reporter transcripts described in Figure 2C (top panel). In control experiments, EPRS and L13a replaced phospho-EPRS and phospho-L13a, respectively. Luc expression was quantitated by densitometry, normalized by T7 gene 10 expression, and reported as mean ± s.e.m. (n = 4 experiments) (2nd panel). The experimental condition that reconstituted translational silencing is denoted with an asterisk (P<0.001, two-tailed T-test). The stability of Luc and T7 gene 10 reporter mRNAs was determined as in Figure 2C (3rd through 6th panels).
Figure 6. L13a and GAPDH Direct a Conformational Switch that Derepresses GAIT Element Binding to EPRS
(A) Schematic showing YFP-labeled EPRS linker and CFP-labeled NSAP1 used as probes in FRET analysis of conformational switch. (B) Conformational switch determined by FRET. U937 cells expressing YFP-EPRS linker and CFP-NSAP1 (left) were treated with IFN-γ for 0 (×), 8 (□), and 24 (○) h, and CFP and YFP emission spectra were collected. Background from non-transfected cell lysate was subtracted, and spectra were normalized to CFP emission of a reference spectrum. The distance between probes was calculated from FRET efficiency by the Förster equation (inset table). (C) Absence of conformational switch in cells lacking L13a. Spectra were obtained from cells transfected with shRNA targeted against L13a, as in (B). Immunoblot with anti-L13a indicates effective depletion (upper inset). The distance between probes was calculated from FRET efficiency (inset table). (D) Model of dual function of WHEP domains in GAIT complex assembly. EPRS phosphorylation induces its release from the tRNA multisynthetase complex (left). After 2–4 h, NSAP1 binds to phospho-EPRS linker domain R2R3 and blocks GAIT element binding to domain R1R2 (center). After 12–16 h, joining of phospho-L13a and GAPDH releases the inhibition by NSAP1, possibly by inducing a conformational change or relocalization, and permits GAIT element binding to EPRS domain R1R2 (right).
Comment in
- Not just because it is there: aminoacyl-tRNA synthetases gain control of the cell.
Ribas de Pouplana L, Geslain R. Ribas de Pouplana L, et al. Mol Cell. 2008 Apr 11;30(1):3-4. doi: 10.1016/j.molcel.2008.03.006. Mol Cell. 2008. PMID: 18406320
Similar articles
- The GAIT translational control system.
Arif A, Yao P, Terenzi F, Jia J, Ray PS, Fox PL. Arif A, et al. Wiley Interdiscip Rev RNA. 2018 Mar;9(2):e1441. doi: 10.1002/wrna.1441. Epub 2017 Nov 20. Wiley Interdiscip Rev RNA. 2018. PMID: 29152905 Free PMC article. Review. - Heterotrimeric GAIT complex drives transcript-selective translation inhibition in murine macrophages.
Arif A, Chatterjee P, Moodt RA, Fox PL. Arif A, et al. Mol Cell Biol. 2012 Dec;32(24):5046-55. doi: 10.1128/MCB.01168-12. Epub 2012 Oct 15. Mol Cell Biol. 2012. PMID: 23071094 Free PMC article. - Two-site phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity.
Arif A, Jia J, Mukhopadhyay R, Willard B, Kinter M, Fox PL. Arif A, et al. Mol Cell. 2009 Jul 31;35(2):164-80. doi: 10.1016/j.molcel.2009.05.028. Mol Cell. 2009. PMID: 19647514 Free PMC article. - The GAIT system: a gatekeeper of inflammatory gene expression.
Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. Mukhopadhyay R, et al. Trends Biochem Sci. 2009 Jul;34(7):324-31. doi: 10.1016/j.tibs.2009.03.004. Epub 2009 Jun 15. Trends Biochem Sci. 2009. PMID: 19535251 Free PMC article. Review. - Evolution of function of a fused metazoan tRNA synthetase.
Ray PS, Sullivan JC, Jia J, Francis J, Finnerty JR, Fox PL. Ray PS, et al. Mol Biol Evol. 2011 Jan;28(1):437-47. doi: 10.1093/molbev/msq246. Epub 2010 Sep 9. Mol Biol Evol. 2011. PMID: 20829344 Free PMC article.
Cited by
- Essential nontranslational functions of tRNA synthetases.
Guo M, Schimmel P. Guo M, et al. Nat Chem Biol. 2013 Mar;9(3):145-53. doi: 10.1038/nchembio.1158. Nat Chem Biol. 2013. PMID: 23416400 Free PMC article. Review. - Origin and evolution of glutamyl-prolyl tRNA synthetase WHEP domains reveal evolutionary relationships within Holozoa.
Ray PS, Fox PL. Ray PS, et al. PLoS One. 2014 Jun 26;9(6):e98493. doi: 10.1371/journal.pone.0098493. eCollection 2014. PLoS One. 2014. PMID: 24968216 Free PMC article. - Disease-associated mutations in a bifunctional aminoacyl-tRNA synthetase gene elicit the integrated stress response.
Jin D, Wek SA, Kudlapur NT, Cantara WA, Bakhtina M, Wek RC, Musier-Forsyth K. Jin D, et al. J Biol Chem. 2021 Oct;297(4):101203. doi: 10.1016/j.jbc.2021.101203. Epub 2021 Sep 17. J Biol Chem. 2021. PMID: 34537243 Free PMC article. - Condensin II and GAIT complexes cooperate to restrict LINE-1 retrotransposition in epithelial cells.
Ward JR, Vasu K, Deutschman E, Halawani D, Larson PA, Zhang D, Willard B, Fox PL, Moran JV, Longworth MS. Ward JR, et al. PLoS Genet. 2017 Oct 13;13(10):e1007051. doi: 10.1371/journal.pgen.1007051. eCollection 2017 Oct. PLoS Genet. 2017. PMID: 29028794 Free PMC article. - Recognition of the tRNA structure: Everything everywhere but not all at once.
Zhang J. Zhang J. Cell Chem Biol. 2024 Jan 18;31(1):36-52. doi: 10.1016/j.chembiol.2023.12.008. Epub 2023 Dec 29. Cell Chem Biol. 2024. PMID: 38159570 Free PMC article. Review.
References
- Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, et al. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am. J. Hum. Genet. 2003;72:1293–1299. - PMC - PubMed
- Berglund H, Rak A, Serganov A, Garber M, Härd T. Solution structure of the ribosomal RNA binding protein S15 from Thermus thermophilus. Nat. Struct. Biol. 1997;4:20–23. - PubMed
- Berthonneau E, Mirande M. A gene fusion event in the evolution of aminoacyl-tRNA synthetases. FEBS Lett. 2000;470:300–304. - PubMed
- Blanc V, Navaratnam N, Henderson JO, Anant S, Kennedy S, Jarmuz A, Scott J, Davidson NO. Identification of GRY-RBP as an apolipoprotein B RNA-binding protein that interacts with both apobec-1 and apobec-1 complementation factor to modulate C to U editing. J. Biol. Chem. 2001;276:10272–10283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL076491/HL/NHLBI NIH HHS/United States
- P01 HL029582/HL/NHLBI NIH HHS/United States
- R01 HL67725/HL/NHLBI NIH HHS/United States
- P01 HL29582/HL/NHLBI NIH HHS/United States
- R01 HL067725/HL/NHLBI NIH HHS/United States
- P01 HL76491/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous