Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction - PubMed (original) (raw)
Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction
Ok S Shin et al. Infect Immun. 2008 Jun.
Abstract
The contribution of Toll-like receptors (TLRs) to phagocytosis of Borrelia burgdorferi has not been extensively studied. We show that bone marrow-derived macrophages (BMDM) from MyD88(-/-) mice or Raw cells transfected with a dominant-negative MyD88 were unable to efficiently internalize B. burgdorferi. Knockouts of TLR2 and TLR9 or knockdown of TLR5 by small interfering RNA produced no defects in phagocytosis of B. burgdorferi. Production of inflammatory cytokines was greatly diminished in MyD88(-/-) BMDM but only partially affected in TLR2(-/-) BMDM or knockdown of TLR5 and unaffected in TLR9(-/-) BMDM. Cytochalasin D reduced cytokine induction, but not to the level of the MyD88(-/-) BMDM. Addition of cytochalasin D to TLR2(-/-) BMDM inhibited inflammatory responses to B. burgdorferi to the level of MyD88(-/-) BMDM, consistent with a role for TLR2 in both recognition of extracellular products and lysosomal sampling by TLR2 after processing of the organism. Cytochalasin D had no impact on cytokine production in cells undergoing TLR5 knockdown. These results suggest that MyD88, but not TLR2, TLR5, and TLR9, is important for the uptake of B. burgdorferi and that MyD88 affects inflammatory responses through both its effects on phagocytosis and its role in transducing signals from TLR2 and TLR5.
Figures
FIG. 1.
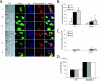
MyD88−/− BMDM show deficient uptake of B. burgdorferi. (A) BMDM from wild-type littermates and MyD88−/− mice were isolated. B. burgdorferi spirochetes were added to the BMDM at an MOI of 10. The cells were incubated with B. burgdorferi for 5, 20, or 60 min. Cells were fixed at each time point and then probed with various antibodies. Lanes: DIC, differential interference contrast; LAMP-1, anti-LAMP-1 (lysosomal marker) antibody (green); Bb, rabbit anti-B. burgdorferi antibody prior to permeabilization of the cells to identify extracellular organisms (blue); Bb (permeabilized), rabbit anti-B. burgdorferi antibody after permeabilization of the cells to identify both internalized and extracellular B. burgdorferi epitopes (red). Lane Merge shows a merged image of LAMP-1, Bb, and Bb (permeabilized). Experiments were repeated five times with cells from two different mice per experiment, and representative images are shown. Scale bar, 10 μM. (B) Cells containing internalized B. burgdorferi particles were counted and expressed as a percentage of the total number of cells examined. (C) Cells containing spirochetes that appeared intact and not degraded (i.e., that maintained their spirochetal architecture) were counted and expressed as a percentage of the total cells. Each experiment was repeated five times using cells from two different mice each time. The error bars represent standard deviations. *, P ≤ 0.05 compared with wild-type (WT) cells at matched postinfection time points. (D) For quantitative analysis of B. burgdorferi survival in the BMDM from wild-type littermates and MyD88−/− mice, B. burgdorferi spirochetes were incubated with macrophages as before. After 1 h of incubation, the cells were washed three times with cold PBS to eliminate unbound B. burgdorferi spirochetes. Cells (106) were resuspended in BSK liquid medium and serially diluted in 96-well plates. The wells were monitored for growth of B. burgdorferi. Experiments were performed in duplicate and repeated three times with cells from two different mice per experiment. Each bar represents one independent experiment.
FIG. 2.
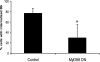
Transient transfection of a MyD88 DN plasmid in Raw 264.7 cells reduces the uptake of B. burgdorferi. Raw 264.7 cells were transiently transfected with pCDNA3-GFP control or MyD88 DN plasmid. After 24 h, the cells were incubated with B. burgdorferi (Bb) for 60 min. Fixed cells were incubated with different fluorescently labeled antibodies as described in the legend to Fig. 1 and then visualized by fluorescence microscopy. Cells containing internalized B. burgdorferi particles at 1 h postinfection were counted and expressed as a percentage of the total number of cells examined. The data are representative of three independent experiments. The error bars represent standard deviations. *, P ≤ 0.05 compared with control-transfected cells.
FIG. 3.
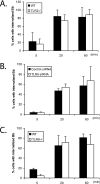
TLR2, TLR5, and TLR9 do not participate in the phagocytosis of B. burgdorferi. (A) Wild-type (WT) control or TLR2−/− BMDM were plated in 24-well plates and infected with B. burgdorferi spirochetes (Bb) for 5 min, 20 min, and 60 min. (B) Raw 264.7 cells were transfected with either control or TLR5 siRNAs, and after 24 h, the cells were infected with B. burgdorferi for 5 min, 20 min, and 60 min. (C) Wild-type control or TLR9−/− BMDM were plated in 24-well plates and infected with B. burgdorferi for 5 min, 20 min, and 60 min. The phagocytosis assay and staining were performed as described in the legend to Fig. 1. Cells containing internalized B. burgdorferi particles were counted and expressed as a percentage of the total number of cells examined. The data are representative of three or four independent experiments. The error bars represent standard deviations.
FIG. 4.
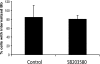
p38 MAPK is not involved in the phagocytosis of B. burgdorferi. Wild-type BMDM were preincubated with either a vehicle control or 10 μM p38 MAPK inhibitor SB203580 for 1 h prior to the addition of B. burgdorferi (Bb) (MOI = 10). The phagocytosis assay and immunofluorescence staining were performed as described in the legend to Fig. 1. Cells containing internalized B. burgdorferi particles at 1 h after B. burgdorferi infection were counted and expressed as a percentage of the total number of cells examined. The data are representative of three independent experiments. The error bars represent standard deviations.
FIG. 5.
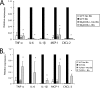
Both MyD88 and TLR2 regulate _B. burgdorferi_-induced expression of inflammatory molecules from BMDM. Wild-type (WT), MyD88−/−, and TLR2−/− BMDM were infected with B. burgdorferi (Bb) at an MOI of 10 for 24 h, and transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR as described in Materials and Methods. MyD88−/− and littermate wild-type control macrophages are shown in panel A, and TLR2−/− and wild-type control BMDM are shown in panel B. The expression of target genes was normalized to that of β-actin. The results shown are from three independent experiments performed in duplicate. The expression from wild-type cells infected with B. burgdorferi was arbitrarily set to 1 for all experiments, and the other values are shown relative to that expression level. The error bars represent standard deviations. *, P ≤ 0.05 compared to wild-type cells infected with B. burgdorferi.
FIG. 6.
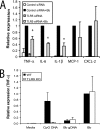
TLR5, but not TLR9, is involved in inflammatory signaling in response to B. burgdorferi. (A) Raw 264.7 cells were transfected with either control or TLR5-specific siRNAs; 24 h later, the cells were stimulated with either medium or B. burgdorferi for 24 h. Transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR, as described in Materials and Methods. Expression with cells transfected with control siRNA and infected with B. burgdorferi was arbitrarily set to 1 for all experiments, and the other values are shown relative to that expression level. (B) Wild-type (WT) and TLR9−/− BMDM were collected and plated in six-well plates. The BMDM were stimulated with either 200 nM CpG DNA, 1 μg purified B. burgdorferi genomic DNA (Bb gDNA), or whole B. burgdorferi spirochetes (Bb) for 4 h, and cells were harvested for RNA isolation. Transcriptional expression of TNF-α is shown. The expression of cytokines in wild-type BMDM infected with B. burgdorferi was arbitrarily set to 1. The real-time PCR experiments were performed in duplicate and repeated three times, and the average of all experiments is shown. The error bars represent standard deviations. *, P ≤ 0.05 compared to control siRNA stimulated with B. burgdorferi. KO, knockout.
FIG. 7.
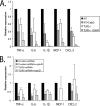
Effects of inhibition of phagocytosis by cytochalasin D on inflammatory signaling via TLR2 and TLR5. (A) BMDM from either wild-type (WT) control or TLR2−/− mice were plated in six-well plates. Cytochalasin D (1 μM) was added to the macrophages 1 h before incubation with B. burgdorferi to block uptake of the bacteria into BMDM. The BMDM were stimulated with B. burgdorferi for 24 h, and cells were harvested for RNA isolation. Transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR, as described in Materials and Methods. The expression of target genes was normalized to that of β-actin. Expression with wild-type cells infected with B. burgdorferi was arbitrarily set to 1 for all the experiments, and the other values are shown relative to that expression level. The experiments were performed three times in duplicate, and the averages of the experiments are shown. The error bars represent standard deviations. The P values for all tested cytokines and chemokines, comparing _B. burgdorferi_-infected wild-type and TLR2−/− BMDM, were ≤0.05. Comparisons between _B. burgdorferi_-infected wild-type cells treated or not with cytochalasin D and between _B. burgdorferi_-infected wild-type cells and TLR2−/− cells treated with cytochalasin D were also all statistically significant (P ≤ 0.05). (B) Raw 264.7 cells were transfected with either control or TLR5 siRNAs. After 24 h, cytochalasin D (1 μΜ) was added to the macrophages 1 h before incubation with B. burgdorferi to block uptake of the bacteria into the Raw 264.7 cells. The cells were stimulated with B. burgdorferi for 24 h and harvested for RNA isolation. Transcriptional expression of TNF-α, IL-6, IL-1β, MCP-1, and CXCL-2 was measured by qRT-PCR, as described in Materials and Methods. The expression of target genes was normalized to that of β-actin. Expression with control siRNA-transfected cells infected with B. burgdorferi was arbitrarily set to 1 for all the experiments, and the other values are shown relative to that expression level. The experiments were performed three times in duplicate, and the averages of the experiments are shown. The error bars represent standard deviations.
Similar articles
- TRIF mediates Toll-like receptor 2-dependent inflammatory responses to Borrelia burgdorferi.
Petnicki-Ocwieja T, Chung E, Acosta DI, Ramos LT, Shin OS, Ghosh S, Kobzik L, Li X, Hu LT. Petnicki-Ocwieja T, et al. Infect Immun. 2013 Feb;81(2):402-10. doi: 10.1128/IAI.00890-12. Epub 2012 Nov 19. Infect Immun. 2013. PMID: 23166161 Free PMC article. - Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice.
Liu N, Montgomery RR, Barthold SW, Bockenstedt LK. Liu N, et al. Infect Immun. 2004 Jun;72(6):3195-203. doi: 10.1128/IAI.72.6.3195-3203.2004. Infect Immun. 2004. PMID: 15155621 Free PMC article. - The TLR2 ligand FSL-1 and the TLR5 ligand Flagellin mediate pro-inflammatory and pro-labour response via MyD88/TRAF6/NF-κB-dependent signalling.
Lim R, Barker G, Lappas M. Lim R, et al. Am J Reprod Immunol. 2014 May;71(5):401-17. doi: 10.1111/aji.12229. Epub 2014 Mar 17. Am J Reprod Immunol. 2014. PMID: 24635133 - Toll-like receptors as adjuvant receptors.
Kaisho T, Akira S. Kaisho T, et al. Biochim Biophys Acta. 2002 Feb 13;1589(1):1-13. doi: 10.1016/s0167-4889(01)00182-3. Biochim Biophys Acta. 2002. PMID: 11909637 Review. - Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses.
Huang X, Yang Y. Huang X, et al. Expert Opin Ther Targets. 2010 Aug;14(8):787-96. doi: 10.1517/14728222.2010.501333. Expert Opin Ther Targets. 2010. PMID: 20560798 Free PMC article. Review.
Cited by
- Mechanisms of Borrelia burgdorferi internalization and intracellular innate immune signaling.
Petnicki-Ocwieja T, Kern A. Petnicki-Ocwieja T, et al. Front Cell Infect Microbiol. 2014 Dec 15;4:175. doi: 10.3389/fcimb.2014.00175. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25566512 Free PMC article. Review. - The Jarisch-Herxheimer Reaction After Antibiotic Treatment of Spirochetal Infections: A Review of Recent Cases and Our Understanding of Pathogenesis.
Butler T. Butler T. Am J Trop Med Hyg. 2017 Jan 11;96(1):46-52. doi: 10.4269/ajtmh.16-0434. Epub 2016 Oct 24. Am J Trop Med Hyg. 2017. PMID: 28077740 Free PMC article. Review. - Novel microbial virulence factor triggers murine lyme arthritis.
Yang X, Qin J, Promnares K, Kariu T, Anderson JF, Pal U. Yang X, et al. J Infect Dis. 2013 Mar 15;207(6):907-18. doi: 10.1093/infdis/jis930. Epub 2013 Jan 9. J Infect Dis. 2013. PMID: 23303811 Free PMC article. - For Whom the Bell Tolls (and Nods): Spit-acular Saliva.
Shaw DK, Kotsyfakis M, Pedra JH. Shaw DK, et al. Curr Trop Med Rep. 2016 Jun;3(2):40-50. doi: 10.1007/s40475-016-0072-4. Epub 2016 Apr 5. Curr Trop Med Rep. 2016. PMID: 27547699 Free PMC article. - Borrelia burgdorferi lipoprotein BmpA activates pro-inflammatory responses in human synovial cells through a protein moiety.
Yang X, Izadi H, Coleman AS, Wang P, Ma Y, Fikrig E, Anguita J, Pal U. Yang X, et al. Microbes Infect. 2008 Oct;10(12-13):1300-8. doi: 10.1016/j.micinf.2008.07.029. Epub 2008 Aug 5. Microbes Infect. 2008. PMID: 18725314 Free PMC article.
References
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. - PubMed
- Beck, G., G. S. Habicht, J. L. Benach, J. L. Coleman, R. M. Lysik, and R. F. O'Brien. 1986. A role for interleukin-1 in the pathogenesis of Lyme disease. Zentralbl. Bakteriol. Mikrobiol. Hyg. 263133-136. - PubMed
- Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 741462-1470. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases