A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis - PubMed (original) (raw)
A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis
Dongtao Ren et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Plant recognition of pathogens leads to rapid activation of MPK3 and MPK6, two Arabidopsis mitogen-activated protein kinases (MAPKs), and their orthologs in other species. Here, we report that synthesis of camalexin, the major phytoalexin in Arabidopsis, is regulated by the MPK3/MPK6 cascade. Activation of MPK3/MPK6 by expression of active upstream MAPK kinase (MAPKK) or MAPKK kinase (MAPKKK) was sufficient to induce camalexin synthesis in the absence of pathogen attack. Induction of camalexin by Botrytis cinerea was preceded by MPK3/MPK6 activation, and compromised in mpk3 and mpk6 mutants. Genetic analysis placed the MPK3/MPK6 cascade upstream of PHYTOALEXIN DEFICIENT 2 (PAD2) and PAD3, but independent or downstream of PAD1 and PAD4. Camalexin induction after MPK3/MPK6 activation was preceded by rapid and coordinated up-regulation of multiple genes encoding enzymes in the tryptophan (Trp) biosynthetic pathway, in the conversion of Trp to indole-3-acetaldoxime (IAOx, a branch point between primary and secondary metabolism), and in the camalexin biosynthetic pathway downstream of IAOx. These results indicate that the MPK3/MPK6 cascade regulates camalexin synthesis through transcriptional regulation of the biosynthetic genes after pathogen infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
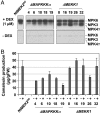
Activation of MPK3 and MPK6 induces camalexin in conditional gain-of-function GVG-NtMEK2 DD transgenic plants. (A) Endogenous MPK3 and MPK6 are required for the full induction of camalexin in GVG-NtMEK2 DD plants. Two-week-old GVG-NtMEK2 DD, GVG-NtMEK2DD/mpk3, and GVG-NtMEK2DD/mpk6 seedlings were treated with 1 μM DEX. Camalexin was quantitated by fluorospectrometry at the indicated times. Error bars indicate standard deviations (n = 3). (B) MPK3 and/or MPK6 activation (Upper), as determined by the in-gel kinase assay, and NtMEK2DD induction (Lower), as determined by immunoblot analysis using anti-Flag antibody. The asterisk indicates a nonspecific band that is recognized by the secondary antibody.
Fig. 2.
Camalexin induction after MPK3/MPK6 activation in conditional gain-of-function MAPKKK transgenic plants. (A) Activation of endogenous MAPKs by constitutively active ΔMEKK1 and ΔMAPKKKα. Five-day-old hygromycin-resistant T2 seedlings were transferred to GC vials. When the seedlings were 2 weeks old, three vials were treated with 1 μM DEX, and three were treated with an equal volume of ethanol as controls (−DEX). Seedlings were collected 24 h later, and MAPK activation was determined by the in-gel kinase assay. (B) Activation of MPK3/MPK6 by ΔMEKK1 and ΔMAPKKKα leads to camalexin accumulation. After the seedlings were collected, camalexin accumulation in the medium was quantitated by fluorospectrometry. Bars represent means and standard deviations (n = 3).
Fig. 3.
Function of MPK3 and MPK6 in pathogen-induced camalexin biosynthesis and resistance against B. cinerea. (A) MAPK activation in Arabidopsis after B. cinerea inoculation. Two-week-old WT (Col-0), mpk3, mpk6, and rescued mpk3/mpk6 double-mutant seedlings were inoculated with Botrytis spores. At the indicated times, seedlings were collected for in-gel kinase assay. (B) Camalexin in the medium was quantitated by fluorospectrometry. Error bars indicate standard deviations (n = 3). (C) Five-week-old soil-grown Arabidopsis plants were inoculated with 10 μl of spore suspension (1 × 105 spores per milliliter). Disease symptoms were scored 3 days later. More than 20 plants per genotype were assayed in each of three independent experiments. Representative images are shown.
Fig. 4.
PAD2 and PAD3, but not PAD1 and PAD4, are required for camalexin induction in GVG-NtMEK2 DD plants. (A) Two-week-old seedlings homozygous for the GVG-NtMEK2 DD transgene and pad1, pad2, pad3, or pad4 were treated with 1 μM DEX, samples were taken at the indicated times, and camalexin levels in the culture medium were determined by fluorospectrometry. Bars represent means and standard deviations (n = 3). (B) Induction of PAD3 expression in pad mutants after MPK3/MPK6 activation, as determined by RNA blot analysis (Upper). Equal loading was confirmed by ethidium bromide (EtBr)-staining of the gel (Lower).
Fig. 5.
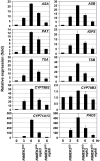
Coordinated induction of Trp biosynthetic genes and cytochrome P450 genes in the camalexin pathway after MPK3/MPK6 activation. Total RNA was extracted from GVG-NtMEK2 DD seedlings before (0 h) and at 3 and 6 h after DEX treatment and from GVG-NtMEK2DD/mpk3 and GVG-NtMEK2DD/mpk6 seedlings 6 h after DEX treatment. After reverse transcription, the levels of each gene were determined by real-time qRT-PCR analysis. The comparative Ct method was used to calculate the levels of transcripts relative to that in GVG-NtMEK2 DD plants without DEX treatment (0 h), which was set at 1. Levels of _EF1_α transcript were used to normalize different samples. Bars represent means and standard deviations (n = 3).
Fig. 6.
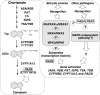
A model of the role of the MAPKKKα/MEKK1–MKK4/MKK5–MPK3/MPK6 cascade in regulating camalexin biosynthesis in plants challenged by pathogens. A simplified camalexin biosynthetic pathway and its regulatory pathway are placed in separate rectangular boxes with dashed outlines. Genes identified by genetic screens (PAD1 to PAD4) are boxed. Enzymes whose encoding genes are induced by pathogen infection and MPK3/MPK6 activation are marked by bold font. One arrow may represent multiple steps because of unknown components.
Similar articles
- Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis.
Mao G, Meng X, Liu Y, Zheng Z, Chen Z, Zhang S. Mao G, et al. Plant Cell. 2011 Apr;23(4):1639-53. doi: 10.1105/tpc.111.084996. Epub 2011 Apr 15. Plant Cell. 2011. PMID: 21498677 Free PMC article. - Differential Phosphorylation of the Transcription Factor WRKY33 by the Protein Kinases CPK5/CPK6 and MPK3/MPK6 Cooperatively Regulates Camalexin Biosynthesis in Arabidopsis.
Zhou J, Wang X, He Y, Sang T, Wang P, Dai S, Zhang S, Meng X. Zhou J, et al. Plant Cell. 2020 Aug;32(8):2621-2638. doi: 10.1105/tpc.19.00971. Epub 2020 May 21. Plant Cell. 2020. PMID: 32439826 Free PMC article. - Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis.
Zhou J, Mu Q, Wang X, Zhang J, Yu H, Huang T, He Y, Dai S, Meng X. Zhou J, et al. Plant Cell. 2022 Jul 30;34(8):3066-3087. doi: 10.1093/plcell/koac139. Plant Cell. 2022. PMID: 35543483 Free PMC article. - Camalexin.
Glawischnig E. Glawischnig E. Phytochemistry. 2007 Feb;68(4):401-6. doi: 10.1016/j.phytochem.2006.12.005. Epub 2007 Jan 10. Phytochemistry. 2007. PMID: 17217970 Review. - The role of phytoalexins in plant protection.
Hammerschmidt R, Dann EK. Hammerschmidt R, et al. Novartis Found Symp. 1999;223:175-87; discussion 188-90. doi: 10.1002/9780470515679.ch12. Novartis Found Symp. 1999. PMID: 10549555 Review.
Cited by
- Stress-responsive mitogen-activated protein kinases interact with the EAR motif of a poplar zinc finger protein and mediate its degradation through the 26S proteasome.
Hamel LP, Benchabane M, Nicole MC, Major IT, Morency MJ, Pelletier G, Beaudoin N, Sheen J, Séguin A. Hamel LP, et al. Plant Physiol. 2011 Nov;157(3):1379-93. doi: 10.1104/pp.111.178343. Epub 2011 Aug 26. Plant Physiol. 2011. PMID: 21873571 Free PMC article. - Glutathione-indole-3-acetonitrile is required for camalexin biosynthesis in Arabidopsis thaliana.
Su T, Xu J, Li Y, Lei L, Zhao L, Yang H, Feng J, Liu G, Ren D. Su T, et al. Plant Cell. 2011 Jan;23(1):364-80. doi: 10.1105/tpc.110.079145. Epub 2011 Jan 14. Plant Cell. 2011. PMID: 21239642 Free PMC article. - Mitogen-activated protein kinase phosphatase 1 reduces the replication efficiency of Bamboo mosaic virus in Nicotiana benthamiana.
Lee CC, Wu YJ, Hsueh CH, Huang YT, Hsu YH, Meng M. Lee CC, et al. Mol Plant Pathol. 2018 Oct;19(10):2319-2332. doi: 10.1111/mpp.12701. Epub 2018 Aug 2. Mol Plant Pathol. 2018. PMID: 29806182 Free PMC article. - Modifications of Sphingolipid Content Affect Tolerance to Hemibiotrophic and Necrotrophic Pathogens by Modulating Plant Defense Responses in Arabidopsis.
Magnin-Robert M, Le Bourse D, Markham J, Dorey S, Clément C, Baillieul F, Dhondt-Cordelier S. Magnin-Robert M, et al. Plant Physiol. 2015 Nov;169(3):2255-74. doi: 10.1104/pp.15.01126. Epub 2015 Sep 16. Plant Physiol. 2015. PMID: 26378098 Free PMC article. - The Vacuolar Proton-Cation Exchanger EcNHX1 Generates pH Signals for the Expression of Secondary Metabolism in Eschscholzia californica.
Weigl S, Brandt W, Langhammer R, Roos W. Weigl S, et al. Plant Physiol. 2016 Feb;170(2):1135-48. doi: 10.1104/pp.15.01570. Epub 2015 Nov 17. Plant Physiol. 2016. PMID: 26578709 Free PMC article.
References
- Boller T. Peptide signalling in plant development and self/non-self perception. Cur Opin Cell Biol. 2005;17:116–122. - PubMed
- Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nat Immunol. 2005;6:973–979. - PubMed
- Dangl JL, Jones JDG. Plant pathogens and integrated defense responses to infection. Nature. 2001;411:826–833. - PubMed
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JDG. Molecular genetics of plant disease resistance. Science. 1995;268:661–667. - PubMed
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases