A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1 - PubMed (original) (raw)
Comparative Study
A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1
Nathaniel A Jeske et al. Pain. 2008.
Abstract
Certain phosphorylation events are tightly controlled by scaffolding proteins such as A-kinase anchoring protein (AKAP). On nociceptive terminals, phosphorylation of transient receptor potential channel type 1 (TRPV1) results in the sensitization to many different stimuli, contributing to the development of hyperalgesia. In this study, we investigated the functional involvement of AKAP150 in mediating sensitization of TRPV1, and found that AKAP150 is co-expressed in trigeminal ganglia (TG) neurons from rat and associates with TRPV1. Furthermore, siRNA-mediated knock-down of AKAP150 expression led to a significant reduction in PKA phosphorylation of TRPV1 in cultured TG neurons. In CHO cells, the PKA RII binding site on AKAP was necessary for PKA enhancement of TRPV1-mediated Ca2+-accumulation. In addition, AKAP150 knock-down in cultured TG neurons attenuated PKA sensitization of TRPV1 activity and in vivo administration of an AKAP antagonist significantly reduced prostaglandin E2 sensitization to thermal stimuli. These data suggest that AKAP150 functionally regulates PKA-mediated phosphorylation/sensitization of the TRPV1 receptor.
Figures
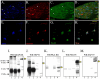
Figure 1. TRPV1, AKAP150 and PKA RII co-express and co-immunoprecipitate in TG Neurons
A-D.) Immunohistochemical co-expression of TRPV1 (blue), AKAP150 (red) and PKA RII (green) in intact TG neurons excised from rat, scale bar in yellow represents 50 μm. E-H.) Immunocytochemical co-expression of TRPV1 (blue), AKAP150 (red) and PKA RII (green) in cultured TG neurons from rat, scale bar in yellow represents 50 μm. I-M.) Co-immunoprecipitation of proteins from cultured T neurons. Lane 1:AKAP150 immunoprecipitation, lane 2: TRPV1 immunoprecipitation, lane 3: 50 μg TG neuron cell lysate, lane 4: 50 μg brain homogenate. Blots were probed for (left to right) AKAP150 (I), TRPV1 (J), PKA RII (K), PSD-95 (L), SAP-97 (M).
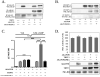
Figure 2. siRNA directed knock-down of AKAP150 expression in TG neurons
A.) Cultured TG neurons transfected with nothing (−), mock transfected (Mock), scrambled/control negative silencer #2 siRNA (Scram), AKAP150-1 siRNA (AKAP-1), or AKAP150-2 siRNA (AKAP-2). Western blot expression of AKAP150 (above) following transfection quantified and normalized to expression of TRPV1. B.) Cultured TG neurons transfected with AKAP150-1 harvested day 0, 1, 2, 3 or 4 following transfection. Western blot expression of AKAP150 (above) following transfection quantified and normalized to expression of TRPV1. C.) Real-time RT-PCR experiments were performed with RNA samples from cultured TG neurons left alone (control), mock transfected (Mock), transfected with scrambled/control negative silencer #2 control siRNA (Scram), or with AKAP150-1 (AKAP-1 siRNA) following 1 day post-transfection. Reactions were performed using primers specific for AKAP150 (PRKA) gene and the internal control (18S). Data were normalized to the relative amount of control AKAP150 mRNA/18S. Data are presented as mean ± SEM (n = 6 per group). D.) Cultured TG neurons mock transfected (Mock), with scrambled/control negative silencer #2 control siRNA (Scram), or with AKAP150-1 (AKAP-1) were analyzed for 32P-incorporation following vehicle (H20) or 8-Br-cAMP (10 μM, 5 min). Autoradiographic results of labeled TRPV1 quantified and normalized to total TRPV1 expression. E.) Cultured TG neurons mock transfected or with AKAP150-1 siRNA were surface biotinylated and analyzed for TRPV1 plasma membrane expression relative to total cell expression of AKAP150. All plotted data are expressed as mean ± SE, **p<0.01, ***p<0.005, NS = not significant.
Figure 3. PKA association with AKAP150 in CHO is necessary for PKA sensitization of TRPV1 activity
A.) Co-immunoprecipitation of AKAP150, with Western blot analysis of association with TRPV1 and PKA RII in co-transfected CHO cells. B.) Co-immunoprecipitation of TRPV1, with Western blot analysis of association with AKAP150 and PKA RII in co-transfected CHO cells. C.) Calcium imaging of CHO cells transfected with AKAP150, TRPV1, and/or AKAP150ΔPKA followed by pretreatment with either vehicle or 8-Br-cAMP (10 μM, 5 min), followed by stimulation of all groups with capsaicin (100 nM). D.) Basal in vitro activity of immunoprecipitated PKA from co-transfected CHO cells, with Western blot analysis of AKAP150, TRPV1 and PKA RII from cell lysates to demonstrate equal protein expression. All plotted data are expressed as mean ± SE, ***p<0.005.
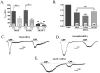
Figure 4. Pharmacological desensitization of TRPV1 in sensory neurons is independent of AKAP150 expression
A.) Cultured TG neurons mock transfected (Mock), transfected with scrambled/control negative silencer #2 control siRNA (Scram) or AKAP150-1 (AKAP-1) were analyzed for capsaicin (CAP; 50 nm, 30 sec)-activated current (ICAP) tachyphylaxis, n shown on graph. Veh represents first response to CAP application. B.) ICAP values after second application of CAP (50 nm) normalized to first application of CAP (i.e. control). Recordings taken from cultured TG neurons as in panel A, n shown on graph. C.) Representative trace of recordings taken from cultured TG neurons mock transfected, treatments indicated. D.) Representative trace of recordings taken from cultured TG neurons transfected with scrambled/control negative silencer #2 siRNA (negative control), treatments indicated. E.) Representative trace of recordings taken from cultured TG neurons transfected with AKAP150-1 specific siRNA, treatments indicated. All plotted data are expressed as mean ± SE, *p<0.05, **p<0.01, ***p<0.005, NS = not significant.
Figure 5. PKA sensitization of TRPV1 activity is dependent on AKAP150 expression
A.) Cultured TG neurons transfected as in A, analyzed for ICAP (CAP: 50nm, 30 sec) sensitization following vehicle (H2O) or 8-Br-cAMP (10 μM, 5 min) pre-treatment. Illustrated data normalized to vehicle treatment, n shown on graph. B.) in vitro activity of immunoprecipitated PKA from mock (Mock) or AKAP150-1 (AKAP)-transfected TG neurons following H2O vehicle or 8-Br-cAMP (10 μM , 5 sec) treatment, with Western blot analysis of AKAP150 and PKA RII from cell lysates to demonstrate equal protein expression. C.) Representative trace of recordings taken from cultured TG neurons mock transfected, treatments indicated. D.) Representative trace of recordings taken from cultured TG neurons transfected with scrambled/control negative silencer #2 siRNA (negative control), treatments indicated. E.) Representative trace of recordings taken from cultured TG neurons transfected with AKAP150-1 specific siRNA, treatments indicated. All plotted data are expressed as mean ± SE, **p<0.01, ***p<0.005, NS = not significant.
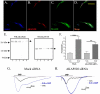
Figure 6. AKAP220 expression in cultured TG neurons
A-D.) Immunohistochemical co-localization of TRPV1 (blue), AKAP220 (red) and PKA RII (green) in cultured TG neurons from rat, scale bar in yellow represents 50 μm. E.) Western blot analysis of AKAP220 and AKAP150 expression from 50 μg TG neuronal lysate following siRNA knock-down: lane 1, no transfection; lane 2, mock; lane 3, AKAP150-1 FITC siRNA; lane 4, AKAP220-1 siRNA; lane 5, AKAP220-2 siRNA. F.) Cultured TG neurons mock transfected (Mock) or transfected with AKAP220-1 siRNA (AKAP220 siRNA) and analyzed for ICAP (50 nm) following vehicle (H20) or 8-Br-cAMP (10 μM) treatment. n=7-12/group. G-H.) Representative trace of recordings taken from cultured TG neurons transfected with mock (G) or AKAP220 (H) siRNA, treatments indicated. All plotted data are expressed as mean ± SE, **p<0.01, ***p<0.005.
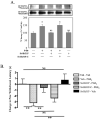
Figure 7. AKAP150/PKA association is necessary for TRPV1 phosphorylation and sensitization
A.) Cultured TG neurons pre-treated with tris/H20 veh, St-HT31P (50 μmol), or StHT31P-C (50 μmol) 15 min, followed by 8-Br-cAMP (10 mM, 5 min), and analyzed for TRPV1 phosphorylation by autoradiographic densitometry normalized to Western blot immunoreactivity. *p<0.05, NS = not significant. B.) Rats were injected with 50 μl Tris/saline veh, St-HT31P (5 μmol), or St-HT31P-C (5 μmol) in the right hindpaw. The same paws were injected with 0.01% ethanol/saline veh or 0.3 μg PGE2 15 min later, upon which thermal paw withdrawal latencies were measured by blinded observers. Data are depicted as change in paw withdrawal latency (sec) from baseline valuations taken before injections, such that PGE2 produces shorter paw withdrawal latency values vs. baseline). All plotted data expressed as mean ± SEM, n= 6–10 animals per group, two-way ANOVA with Bonferroni post hoc test, **p<0.01, NS=not significant.
Similar articles
- Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons.
Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Schnizler K, et al. J Neurosci. 2008 May 7;28(19):4904-17. doi: 10.1523/JNEUROSCI.0233-08.2008. J Neurosci. 2008. PMID: 18463244 Free PMC article. - A-kinase anchoring protein 150 mediates transient receptor potential family V type 1 sensitivity to phosphatidylinositol-4,5-bisphosphate.
Jeske NA, Por ED, Belugin S, Chaudhury S, Berg KA, Akopian AN, Henry MA, Gomez R. Jeske NA, et al. J Neurosci. 2011 Jun 8;31(23):8681-8. doi: 10.1523/JNEUROSCI.0020-11.2011. J Neurosci. 2011. PMID: 21653872 Free PMC article. - A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1.
Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. Jeske NA, et al. Pain. 2009 Dec;146(3):301-307. doi: 10.1016/j.pain.2009.08.002. Epub 2009 Sep 19. Pain. 2009. PMID: 19767149 Free PMC article. - Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia.
Lee J, Chung MK, Ro JY. Lee J, et al. Biochem Biophys Res Commun. 2012 Jul 27;424(2):358-63. doi: 10.1016/j.bbrc.2012.07.008. Epub 2012 Jul 10. Biochem Biophys Res Commun. 2012. PMID: 22789851 Free PMC article. - Proteomic analyses of PKA and AKAP signaling in cocaine addiction.
Reissner KJ. Reissner KJ. Neuropsychopharmacology. 2013 Jan;38(1):251-2. doi: 10.1038/npp.2012.181. Neuropsychopharmacology. 2013. PMID: 23147493 Free PMC article. Review. No abstract available.
Cited by
- Induction of thermal and mechanical hypersensitivity by parathyroid hormone-related peptide through upregulation of TRPV1 function and trafficking.
Mickle AD, Shepherd AJ, Loo L, Mohapatra DP. Mickle AD, et al. Pain. 2015 Sep;156(9):1620-1636. doi: 10.1097/j.pain.0000000000000224. Pain. 2015. PMID: 25970319 Free PMC article. - Exaptation and Evolutionary Adaptation in Nociceptor Mechanisms Driving Persistent Pain.
Walters ET. Walters ET. Brain Behav Evol. 2023;98(6):314-330. doi: 10.1159/000535552. Epub 2023 Nov 30. Brain Behav Evol. 2023. PMID: 38035556 Free PMC article. Review. - Tyrosine phosphorylation tunes chemical and thermal sensitivity of TRPV2 ion channel.
Mo X, Pang P, Wang Y, Jiang D, Zhang M, Li Y, Wang P, Geng Q, Xie C, Du HN, Zhong B, Li D, Yao J. Mo X, et al. Elife. 2022 Jun 10;11:e78301. doi: 10.7554/eLife.78301. Elife. 2022. PMID: 35686730 Free PMC article. - A-kinase anchoring protein 150 expression in a specific subset of TRPV1- and CaV 1.2-positive nociceptive rat dorsal root ganglion neurons.
Brandao KE, Dell'Acqua ML, Levinson SR. Brandao KE, et al. J Comp Neurol. 2012 Jan 1;520(1):81-99. doi: 10.1002/cne.22692. J Comp Neurol. 2012. PMID: 21674494 Free PMC article. - Transient Receptor Potential Vanilloid 1 Signaling Is Independent on Protein Kinase A Phosphorylation of Ankyrin-Rich Membrane Spanning Protein.
Pellegrino A, Mükusch S, Seitz V, Stein C, Herberg FW, Seitz H. Pellegrino A, et al. Med Sci (Basel). 2022 Nov 17;10(4):63. doi: 10.3390/medsci10040063. Med Sci (Basel). 2022. PMID: 36412904 Free PMC article.
References
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24(18):4300–12. - PMC - PubMed
- Bauman GP, Bartik MM, Brooks WH, Roszman TL. Induction of cAMP-dependent protein kinase (PKA) activity in T cells after stimulation of the prostaglandin E2 or the beta-adrenergic receptors: relationship between PKA activity and inhibition of anti-CD3 monoclonal antibody-induced T cell proliferation. Cell Immunol. 1994;158(1):182–94. - PubMed
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35(4):721–31. - PubMed
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE13942/DE/NIDCR NIH HHS/United States
- DE016500/DE/NIDCR NIH HHS/United States
- F32 DE016500-02/DE/NIDCR NIH HHS/United States
- F32 DE016500/DE/NIDCR NIH HHS/United States
- DA19585/DA/NIDA NIH HHS/United States
- R01 DA019585/DA/NIDA NIH HHS/United States
- R01 DE013942/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous