Anesthetics impact the resolution of inflammation - PubMed (original) (raw)
Anesthetics impact the resolution of inflammation
Nan Chiang et al. PLoS One. 2008.
Abstract
Background: Local and volatile anesthetics are widely used for surgery. It is not known whether anesthetics impinge on the orchestrated events in spontaneous resolution of acute inflammation. Here we investigated whether a commonly used local anesthetic (lidocaine) and a widely used inhaled anesthetic (isoflurane) impact the active process of resolution of inflammation.
Methods and findings: Using murine peritonitis induced by zymosan and a systems approach, we report that lidocaine delayed and blocked key events in resolution of inflammation. Lidocaine inhibited both PMN apoptosis and macrophage uptake of apoptotic PMN, events that contributed to impaired PMN removal from exudates and thereby delayed the onset of resolution of acute inflammation and return to homeostasis. Lidocaine did not alter the levels of specific lipid mediators, including pro-inflammatory leukotriene B(4), prostaglandin E(2) and anti-inflammatory lipoxin A(4), in the cell-free peritoneal lavages. Addition of a lipoxin A(4) stable analog, partially rescued lidocaine-delayed resolution of inflammation. To identify protein components underlying lidocaine's actions in resolution, systematic proteomics was carried out using nanospray-liquid chromatography-tandem mass spectrometry. Lidocaine selectively up-regulated pro-inflammatory proteins including S100A8/9 and CRAMP/LL-37, and down-regulated anti-inflammatory and some pro-resolution peptides and proteins including IL-4, IL-13, TGF-â and Galectin-1. In contrast, the volatile anesthetic isoflurane promoted resolution in this system, diminishing the amplitude of PMN infiltration and shortening the resolution interval (Ri) approximately 50%. In addition, isoflurane down-regulated a panel of pro-inflammatory chemokines and cytokines, as well as proteins known to be active in cell migration and chemotaxis (i.e., CRAMP and cofilin-1). The distinct impact of lidocaine and isoflurane on selective molecules may underlie their opposite actions in resolution of inflammation, namely lidocaine delayed the onset of resolution (T(max)), while isoflurane shortened resolution interval (Ri).
Conclusions: Taken together, both local and volatile anesthetics impact endogenous resolution program(s), altering specific resolution indices and selective cellular/molecular components in inflammation-resolution. Isoflurane enhances whereas lidocaine impairs timely resolution of acute inflammation.
Conflict of interest statement
Competing Interests: Brigham and Women's Hospital is assigned patents on lipoxins that are subjects of licensing agreements and consultant arrangements for C.N.S.
Figures
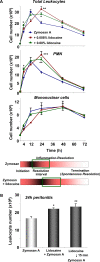
Figure 1. Lidocaine alters leukocyte infiltration during acute inflammation and delays resolution.
(A) Mice were injected with zymosan A in the absence or presence of lidocaine (0.008% or 0.08%) and peritoneal lavages were collected at indicated time points. Total leukocytes were enumerated by light microscopy, and PMN and mononuclear cells determined by differential leukocyte counting. Results are expressed as the mean±SEM from n = 3–4. *p<0.05, **p<0.01, ***p<0.001 when compared to mice treated with zymosan A alone at the same time points. (B) Mice were injected with lidocaine (0.08%) 15 min prior to injection of zymosan A. Peritoneal lavages were collected at 24 h, and total leukocytes enumerated. Results are expressed as mean±SEM from n = 3. *p<0.05, **p<0.01 when compared to mice treated with zymosan A alone.
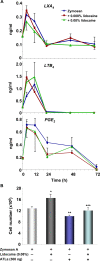
Figure 2. Lidocaine did not directly alter selective eicosanoid levels in cell-free exudates: LXA4 rescues lidocaine-delayed resolution.
(A) Cell-free lavages from murine peritoneum were collected at indicated time points after zymosan challenge (1 mg/ml). LXA4, LTB4 and PGE2 amounts were determined by ELISA. Results are expressed as the mean±SEM from duplicates of n = 3, and were expressed as amounts (ng/ml). (B) Mice were injected with zymosan A together with lidocaine (0.08%), ATLa (300 ng), or lidocaine plus ATLa. Peritoneal lavages were collected at 24 h, and total leukocytes enumerated. Results are expressed as mean±SEM from n = 3. *p = 0.03 **p = 0.01 when compared to mice treated with zymosan A alone. ***p = 0.04 when compared to mice treated with zymosan A and lidocaine.
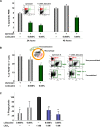
Figure 3. Lidocaine impairs PMN apoptosis and macrophage ingestion of PMN in vivo and zymosan in vitro.
(A) Apoptosis in vivo. Peritoneal cells were collected at 24 h or 48 h and labeled with FITC-annexin-V and PE-conjugated anti-Gr-1 Ab. The apoptotic PMN (annexin-V+Gr-1+) are expressed as % of total PMN (Gr-1+). Results are the mean±SEM from n = 3–4. *p<0.01, **p<0.001. (B) Phagocytosis in vivo. (right) Representative dot plots of FACS analysis. In the non-permeabilized lavage cells, Gr-1+ represents PMN, and F4/80+ represents macrophages; and in the permeabilized cells, F4/80+Gr-1+ cell population represents macrophages with ingested PMN. (left) Results are expressed as the mean±SEM from n = 3–4, and were expressed as percent of the F4/80+Gr-1+ cells. *p<0.05. (C) Phagocytosis in vitro. Murine peritoneal resident macrophages were incubated with indicated compounds or vehicle alone for 20 min followed by addition of FITC-zymosan at a 10:1 ratio for 30 min. Cells were then quenched and fluorescence determined. Phagocytosis activity in the presence of 1 nM of LXA4 was taken as 100%. Results are expressed as the mean±SEM from n = 3–4, and were expressed as % phagocytosis. *p<0.05, **p<0.01, compared to LXA4 alone.
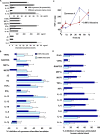
Figure 4. Lidocaine alters pro- and anti-inflammatory proteins: proteomics and cellular proteins.
Mice were injected with zymosan A in the absence or presence of lidocaine. Both lavage fluids (A) and cell pellets (C) were collected at indicated time points and proteins separated by two-dimensional gel electrophoresis. Changes in individual protein levels were measured by image analysis. Selected proteins that display significant differences between treatments are indicated by arrows, and identified by LC/MS/MS and peptide mapping (see Materials and Methods). (B) (Left) S100 A9 protein levels. Supernatants from peritoneal lavages were subjected to Western blot analysis using an anti-S100A9 antibody. Relative intensities of immunoreactive bands were quantitated and normalized by albumin levels using an anti-albumin antibody. Data are expressed as mean±SEM from n = 3–4. *p = 0.02. (Right) S100 A9 mRNA levels. Peritoneal cells were collected and total RNA isolated for RT-PCR analysis using specific primers for mouse S100A9. Relative intensities of RT-PCR products were quantitated and normalized by β-actin message levels. Data are expressed as mean±SEM from n = 3–4. *p<0.01.
Figure 5. Lidocaine regulates selective pro- and anti-inflammatory cytokines/chemokines.
(A, B) Mice were injected with zymosan alone or together with lidocaine (0.008% or 0.08%) for 4 h, and peritoneal cell-free lavage fluids collected. Cytokines and chemokines were expressed as (A) pg/ml or ng/ml in naïve mice and mice treated with zymosan alone, (B) percent inhibition of zymosan A-induced cytokine/chemokine levels by lidocaine. The amounts of cytokines and chemokines levels were determined by multiplexed sandwich ELISA. (C) TGF-β (active form) levels were determined by ELISA. (D) Human heparinized whole blood was incubated with either 0.008% or 0.08% of lidocaine in the presence of zymosan A (100 µg/ml) for 4 h, and plasma was collected. The amounts of cytokines and chemokines levels were determined by multiplexed sandwich ELISA. Results are the mean from duplicate determinations of n = 3–4. *p<0.05 when compared to mice treated with zymosan A alone (B, C) or human whole blood incubated with zymosan A alone (D).
Figure 6. Volatile anesthetic isoflurane reduces leukocyte infiltration and promote resolution by shortening resolution interval.
Mice were administered 1.4 MAC of isoflurane one hour prior to and after injection of zymosan A (1mg/ml, i.p.) (see timeline). The peritoneal lavages were collected at indicated time points. (A) Total leukocytes were enumerated by light microscopy, and PMN and mononuclear cells determined by differential leukocyte counting. Results are expressed as the mean±SEM from n = 3-4. *P<0.05 when compared to mice treated with zymosan A alone at the same intervals. (B) Resolution Indices were calculated with isoflurane as in Figure S1. Isoflurane treatment reduces the magnitude (Ψmax) of inflammation and accelerates resolution by shortening the resolution interval (R_i_.).
Figure 7. Isoflurane regulates cellular proteins-proteomic analysis.
Mice were administered 1.4 Mac of isoflurane one hour prior to and after injection of zymosan A (1mg/ml, i.p.). (A) The peritoneal lavage cells were collected at indicated time points and proteins separated by two-dimensional gel electrophoresis. Changes in individual protein levels were measured by image analysis. Selected proteins that display significant differences between treatments are denoted, and were identified by LC/MS/MS and peptide mapping. (B) Peritoneal cell-free lavage fluids were collected. Cytokine and chemokine levels were determined and expressed as percent inhibition of zymosan A-induced cytokine/chemokine levels by isoflurane. *p<0.05 when compared to mice treated with zymosan A alone. For raw values (pg/ml) of these selective cytokines, see Table 6.
Similar articles
- Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis.
Lee HT, Emala CW, Joo JD, Kim M. Lee HT, et al. Shock. 2007 Apr;27(4):373-9. doi: 10.1097/01.shk.0000248595.17130.24. Shock. 2007. PMID: 17414419 - The pro-apoptotic ARTS protein induces neutrophil apoptosis, efferocytosis, and macrophage reprogramming to promote resolution of inflammation.
Maimon N, Zamir ZZ, Kalkar P, Zeytuni-Timor O, Schif-Zuck S, Larisch S, Ariel A. Maimon N, et al. Apoptosis. 2020 Aug;25(7-8):558-573. doi: 10.1007/s10495-020-01615-3. Apoptosis. 2020. PMID: 32564202 - Molecular and cellular profiles of the resolution phase in a damage-associated molecular pattern (DAMP)-mediated peritonitis model and revelation of leukocyte persistence in peritoneal tissues.
Lastrucci C, Baillif V, Behar A, Al Saati T, Dubourdeau M, Maridonneau-Parini I, Cougoule C. Lastrucci C, et al. FASEB J. 2015 May;29(5):1914-29. doi: 10.1096/fj.14-259341. Epub 2015 Jan 21. FASEB J. 2015. PMID: 25609430 - Resolution therapy: Harnessing efferocytic macrophages to trigger the resolution of inflammation.
Saas P, Vetter M, Maraux M, Bonnefoy F, Perruche S. Saas P, et al. Front Immunol. 2022 Oct 28;13:1021413. doi: 10.3389/fimmu.2022.1021413. eCollection 2022. Front Immunol. 2022. PMID: 36389733 Free PMC article. Review. - The Use and Method of Action of Intravenous Lidocaine and Its Metabolite in Headache Disorders.
Berk T, Silberstein SD. Berk T, et al. Headache. 2018 May;58(5):783-789. doi: 10.1111/head.13298. Epub 2018 Mar 14. Headache. 2018. PMID: 29536530 Review.
Cited by
- Differential general anesthetic effects on microglial cytokine expression.
Ye X, Lian Q, Eckenhoff MF, Eckenhoff RG, Pan JZ. Ye X, et al. PLoS One. 2013;8(1):e52887. doi: 10.1371/journal.pone.0052887. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23382826 Free PMC article. - Isoflurane attenuates sepsis-associated lung injury.
Koutsogiannaki S, Okuno T, Kobayashi Y, Ogawa N, Yuki K. Koutsogiannaki S, et al. Biochem Biophys Res Commun. 2022 Apr 9;599:127-133. doi: 10.1016/j.bbrc.2022.02.028. Epub 2022 Feb 13. Biochem Biophys Res Commun. 2022. PMID: 35180472 Free PMC article. - Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems.
Zhang H, Astrof NS, Liu JH, Wang JH, Shimaoka M. Zhang H, et al. FASEB J. 2009 Aug;23(8):2735-40. doi: 10.1096/fj.09-129908. Epub 2009 Mar 30. FASEB J. 2009. PMID: 19332643 Free PMC article. - MicroRNA-183-5p protects human derived cell line SH-SY5Y cells from mepivacaine-induced injury.
Zhou Q, Zhang L. Zhou Q, et al. Bioengineered. 2021 Dec;12(1):3177-3187. doi: 10.1080/21655979.2021.1946358. Bioengineered. 2021. PMID: 34180760 Free PMC article. - Anesthesia, surgery, illness and Alzheimer's disease.
Eckenhoff RG, Laudansky KF. Eckenhoff RG, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Dec 2;47:162-6. doi: 10.1016/j.pnpbp.2012.06.011. Epub 2012 Jun 21. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 22729032 Free PMC article. Review.
References
- Cotran RS, Kumar V, Collins T, editors. Philadelphia: W.B. Saunders Co; 1999. Robbins Pathologic Basis of Disease. 6th ed. p. 1425.
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-DE016191/DE/NIDCR NIH HHS/United States
- R01 GM038765/GM/NIGMS NIH HHS/United States
- GM 38765/GM/NIGMS NIH HHS/United States
- P50 DE016191/DE/NIDCR NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials