Dual specificity phosphatases 18 and 21 target to opposing sides of the mitochondrial inner membrane - PubMed (original) (raw)
Dual specificity phosphatases 18 and 21 target to opposing sides of the mitochondrial inner membrane
Matthew J Rardin et al. J Biol Chem. 2008.
Abstract
Although large-scale approaches have identified numerous mitochondrial phosphoproteins, little is known about the mitochondrial kinases and phosphatases that regulate these phosphoproteins. Here, we identify two members of the atypical dual specificity phosphatases (DSP), DSP18 and DSP21, that are localized in mitochondria. Although DSP18 is widely expressed in several mammalian tissues, DSP21 is selectively expressed in the testes. We demonstrate that DSP18 and DSP21 are targeted to mitochondria by cryptic internal localization signals. Subfractionation of mitochondria demonstrated that DSP18 is located in the intermembrane space as a peripheral membrane protein of the inner membrane. In contrast, subfractionation of rat testis mitochondria revealed DSP21 is localized to the matrix as a peripheral membrane protein of the inner membrane. Moreover, we demonstrate that a previously reported substrate for DSP18, the stress-activated protein kinase, does not localize to mitochondria in several different tissues, making it an unlikely substrate for DSP18. Finally, we show that induction of apoptosis by treatment with staurosporine causes translocation of DSP18 from the intermembrane space into the cytosol similar to other apoptogenic factors such as cytochrome c. This work rigorously demonstrates the unique location of two highly similar DSPs on opposing sides of the mitochondrial inner membrane.
Figures
FIGURE 1.
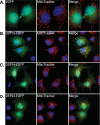
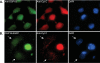
Immunofluorescent analysis of predicted mitochondrial PTPs. Fluorescent images of COS-7 cells transiently transfected for 24 h with constructs encoding EGFP only (A) or C-terminally EGFP tagged DSP18 (B), DSP14 (C), or DSP24 (D). Additionally, cells were stained with the mitochondrial marker, MitoTracker Red, and the nuclear marker, DAPI (blue). Co-localization is represented in the merged images in yellow.
FIGURE 2.
An internal signal sequence directs DSP18 to mitochondria. A, schematic of DSP18 truncations used to assess targeting of EGFP to mitochondria. COS-7 cells transiently expressing C-terminal EGFP-tagged DSP18 (B), 1-94-EGFP (C), 47-141-EGFP (D), 95-188-EGFP (E), and N-terminal EGFP-tagged DSP18. Fluorescent images were taken 24 h post-transfection after co-labeling with MitoTracker Red and DAPI (blue). Co-localization is represented in the merged images in yellow.
FIGURE 3.
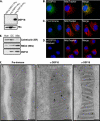
Detection of endogenous DSP18. A, for raising polyclonal antibody, recombinant DSP18-His6 and DSP21-His6 was purified from bacteria, separated by SDS-PAGE, and immunoblotted with protein A-purified anti-DSP18. Blots were stripped and reprobed with an anti-His antibody. Fluorescent images were taken of COS-7 cells probed with anti-DSP18 (B) and pre-immune serum (C), or anti-DSP18 was blocked with recombinant GST-DSP18 (D). B-D, cells were stained with MitoTracker Red and DAPI before visualization. Co-localization is represented in the merged images in yellow. Bars, 10 μm. E, 20 μg of rat kidney homogenate (HOM), differential centrifugation purified mitochondria (DC), and histodenz gradient-purified whole Mito were separated by SDS-PAGE and immunoblotted with anti-calreticulin (endoplasmic reticulum marker), anti-SMAC (mitochondrial marker), and anti-DSP18 antibodies. F, immunogold EM labeling of rat kidney sections (1 and 2) and COS-7 cells transiently expressing DSP18-EGFP (3). Sections were probed with Pre-Immune serum (1) or α-DSP18 antibody (1 and_3_) and labeled with 10-nm gold-conjugated anti-rabbit secondary antibodies. Bar, 100 nm.
FIGURE 4.
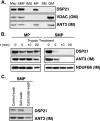
DSP18 is associated with the inner mitochondrial membrane facing the intermembrane space. A, diagram of mitochondrial subfractionation as described under “Experimental Procedures.” B, 20 μg of each submitochondrial fraction (Fig. 4_A_) were separated by SDS-PAGE and immunoblotted with markers for OM (anti-VDAC), IMS (anti-SMAC), IM (anti-PTPMT1), and soluble matrix (SM; anti-Hsp70 - heat shock protein 70). C, 200 μg of MP and SMP were treated with 2.5 μg of trypsin in 100 μl of buffer for the indicated amounts of time. Samples were pelleted, washed, separated by SDS-PAGE, and immunoblotted with anti-DSP18 and anti-PTPMT1. The Complex 1 subunit NDUFB6 was not susceptible to trypsin digestion and was used as a loading control. D, 100 μg of osmotically swollen whole mitochondria and MP were washed with either control buffer, high salt (200 m
m
KCl, 2 m
m
HEPES, pH 7.2), or high pH (0.1
m
Na2CO3, pH 11.5) buffer, repelleted, washed, and separated out by SDS-PAGE. Immunoblots were probed with antibodies against DSP18, cyt c, and PTPMT1.
FIGURE 5.
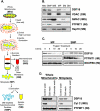
DSP21 is a highly similar phosphatase to DSP18 localized to mitochondria. A, sequence alignment of DSP18 and DSP21, with identities highlighted in gray, and the amino acids of the catalytic triad (D, C, R) highlighted in yellow. The active site residues (PTP-loop; C_X_5R) are highlighted in red. GenBank™ accession numbers are listed next to the sequence. B, COS-7 cells transiently expressing C-terminal EGFP-tagged DSP21. C, fluorescent images were taken of COS-7 cells probed with anti-DSP21. D, COS-7 cells transiently expressing amino acids 43-128 of DSP21 with a C-terminal EGFP tag. B-D, cells were stained with MitoTracker Red and DAPI before visualization. Co-localization is represented in the merged images in yellow. E, schematic of DSP21 truncations used to assess targeting of EGFP to mitochondria. F, 20 μg of rat testis homogenate (HOM), differential centrifugation purified mitochondria (DC), and histodenz gradient purified whole Mito were separated by SDS-PAGE and immunoblotted with anti-calreticulin (endoplasmic reticulum marker), anti-VDAC (mitochondrial marker), and anti-DSP21 antibodies.
FIGURE 6.
DSP21 is a peripheral mitochondrial inner membrane protein facing the matrix. A, 20 μg of each submitochondrial fraction (Fig. 4_A_) were separated by SDS-PAGE and immunoblotted with markers for OM (anti-VDAC), and IM (anti-ANT). B, 200 μg of MP and SMP were treated with 2.5 μg of trypsin in 100 μl of buffer for the indicated amounts of time. Samples were pelleted, washed, separated by SDS-PAGE, and immunoblotted with anti-DSP21 and anti-PTPMT1. The Complex 1 subunit NDUFB6 is not susceptible to trypsin digestion and was used as a loading control. C, 100 μg of SMP were washed with either control buffer, high salt (200 m
m
KCl, 2 m
m
HEPES, pH 7.2), or high pH (0.1
m
Na2CO3, pH 11.5) buffer, repelleted, washed, and separated out by SDS-PAGE. Immunoblots were probed with antibodies against DSP21and ANT.
FIGURE 7.
Endogenous SAPK/JNK is not localized to mitochondria. A, rat kidneys were isolated and homogenized (HOM), nuclei and unbroken cells were removed (post-nuclear supernatant (PNS)), Mito were removed, and the post-mitochondrial supernatant (PMS) was collected. Fractions were separated out by SDS-PAGE and immunoblotted with antibodies against both the p54 and p46 SAPK/JNK isoforms, mitochondrial marker protein Hsp70, and DSP18. B, fractions collected from testis tissue were separated out by SDS-PAGE and immunoblotted with antibodies against SAPK/JNK, Hsp70, and DSP21. C, fractions collected from rat liver tissue were separated out by SDS-PAGE and immunoblotted with antibodies against SAPK/JNK and Hsp70. D, HEK-293A cells were homogenized (Hom), and Mito were isolated by differential centrifugation after treatment with 40 mJ/cm2 of UV radiation and compared with untreated controls. Fractions were separated out and immunoblotted with antibodies against phospho (p)-SAPK/JNK (active), SAPK/JNK, and NDUFB6. E, HEK-293A cells transfected with DSP18-EGFP or a catalytic inactive mutant DSP18(C/S)-EGFP. After 24 h cells were treated with 40 mJ/cm2 of UV radiation, and equal amounts of whole cell lysate were separated out by SDS-PAGE and analyzed for changes in SAPK/JNK phosphorylation.
FIGURE 8.
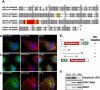
Induction of apoptosis causes translocation of DSP18 from mitochondria into the cytosol. COS-7 cells stained with anti-DSP18 (A) or transfected with DSP18-EGFP (B) were chemically induced to undergo apoptosis. Cells were co-stained with anti-cyt c antibody and DAPI, then visualized by IF for release of cyt c from mitochondria into the cytosol. White arrows (B) indicate a cell not undergoing apoptosis. DAPI staining demonstrates characteristic nuclear condensation and fragmentation.
FIGURE 9.
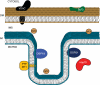
Schematic representation of where the dual specificity phosphatases are localized to in the mitochondria. Bcl-2, B cell lymphoma 2; PTP-MT1, protein-tyrosine phosphatase localized to mitochondria 1. Proteins are not drawn to scale.
Similar articles
- Distinguishing mitochondrial inner membrane orientation of dual specific phosphatase 18 and 21.
Rardin MJ, Taylor GS, Dixon JE. Rardin MJ, et al. Methods Enzymol. 2009;457:275-87. doi: 10.1016/S0076-6879(09)05015-0. Methods Enzymol. 2009. PMID: 19426873 - Structure of human DSP18, a member of the dual-specificity protein tyrosine phosphatase family.
Jeong DG, Cho YH, Yoon TS, Kim JH, Son JH, Ryu SE, Kim SJ. Jeong DG, et al. Acta Crystallogr D Biol Crystallogr. 2006 Jun;62(Pt 6):582-8. doi: 10.1107/S0907444906010109. Epub 2006 May 12. Acta Crystallogr D Biol Crystallogr. 2006. PMID: 16699184 - hNOA1 interacts with complex I and DAP3 and regulates mitochondrial respiration and apoptosis.
Tang T, Zheng B, Chen SH, Murphy AN, Kudlicka K, Zhou H, Farquhar MG. Tang T, et al. J Biol Chem. 2009 Feb 20;284(8):5414-24. doi: 10.1074/jbc.M807797200. Epub 2008 Dec 22. J Biol Chem. 2009. PMID: 19103604 Free PMC article. - Correlated light and electron microscopy illuminates the role of mitochondrial inner membrane remodeling during apoptosis.
Frey TG, Sun MG. Frey TG, et al. Biochim Biophys Acta. 2008 Jul-Aug;1777(7-8):847-52. doi: 10.1016/j.bbabio.2008.05.011. Epub 2008 May 24. Biochim Biophys Acta. 2008. PMID: 18510940 Review. - New insights into the mechanism of precursor protein insertion into the mitochondrial membranes.
Hildenbeutel M, Habib SJ, Herrmann JM, Rapaport D. Hildenbeutel M, et al. Int Rev Cell Mol Biol. 2008;268:147-90. doi: 10.1016/S1937-6448(08)00805-8. Int Rev Cell Mol Biol. 2008. PMID: 18703406 Review.
Cited by
- Redox signaling and protein phosphorylation in mitochondria: progress and prospects.
Foster DB, Van Eyk JE, Marbán E, O'Rourke B. Foster DB, et al. J Bioenerg Biomembr. 2009 Apr;41(2):159-68. doi: 10.1007/s10863-009-9217-7. J Bioenerg Biomembr. 2009. PMID: 19440831 Free PMC article. Review. - Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis.
Zhang J, Guan Z, Murphy AN, Wiley SE, Perkins GA, Worby CA, Engel JL, Heacock P, Nguyen OK, Wang JH, Raetz CR, Dowhan W, Dixon JE. Zhang J, et al. Cell Metab. 2011 Jun 8;13(6):690-700. doi: 10.1016/j.cmet.2011.04.007. Cell Metab. 2011. PMID: 21641550 Free PMC article. - Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria.
Liang H, Ran Q, Jang YC, Holstein D, Lechleiter J, McDonald-Marsh T, Musatov A, Song W, Van Remmen H, Richardson A. Liang H, et al. Free Radic Biol Med. 2009 Aug 1;47(3):312-20. doi: 10.1016/j.freeradbiomed.2009.05.012. Epub 2009 May 15. Free Radic Biol Med. 2009. PMID: 19447173 Free PMC article. - Ptc7p Dephosphorylates Select Mitochondrial Proteins to Enhance Metabolic Function.
Guo X, Niemi NM, Hutchins PD, Condon SGF, Jochem A, Ulbrich A, Higbee AJ, Russell JD, Senes A, Coon JJ, Pagliarini DJ. Guo X, et al. Cell Rep. 2017 Jan 10;18(2):307-313. doi: 10.1016/j.celrep.2016.12.049. Cell Rep. 2017. PMID: 28076776 Free PMC article. - Mitochondrial Enzymes of the Urea Cycle Cluster at the Inner Mitochondrial Membrane.
Haskins N, Bhuvanendran S, Anselmi C, Gams A, Kanholm T, Kocher KM, LoTempio J, Krohmaly KI, Sohai D, Stearrett N, Bonner E, Tuchman M, Morizono H, Jaiswal JK, Caldovic L. Haskins N, et al. Front Physiol. 2021 Jan 21;11:542950. doi: 10.3389/fphys.2020.542950. eCollection 2020. Front Physiol. 2021. PMID: 33551825 Free PMC article.
References
- Hunter, T. (2000) Cell 100 113-127 - PubMed
- Tonks, N. K., and Neel, B. G. (1996) Cell 87 365-368 - PubMed
- Alonso, A., Sasin, J., Bottini, N., Friedberg, I., Friedberg, I., Osterman, A., Godzik, A., Hunter, T., Dixon, J., and Mustelin, T. (2004) Cell 117 699-711 - PubMed
- Visconti, P. E., and Kopf, G. S. (1998) Biol. Reprod. 59 1-6 - PubMed
- Wang, H. G., Rapp, U. R., and Reed, J. C. (1996) Cell 87 629-638 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous