LRRC50, a conserved ciliary protein implicated in polycystic kidney disease - PubMed (original) (raw)
LRRC50, a conserved ciliary protein implicated in polycystic kidney disease
Ellen van Rooijen et al. J Am Soc Nephrol. 2008 Jun.
Abstract
Cilia perform essential motile and sensory functions central to many developmental and physiological processes. Disruption of their structure or function can have profound phenotypic consequences, and has been linked to left-right patterning and polycystic kidney disease. In a forward genetic screen for mutations affecting ciliary motility, we isolated zebrafish mutant hu255H. The mutation was found to disrupt an ortholog of the uncharacterized highly conserved human SDS22-like leucine-rich repeat(LRR)-containing protein LRRC50 (16q24.1) and Chlamydomonas Oda7p. Zebrafish lrrc50 is specifically expressed in all ciliated tissues. lrrc50(hu255H) mutants develop pronephric cysts with an increased proliferative index, severely reduced brush border, and disorganized pronephric cilia manifesting impaired localized fluid flow consistent with ciliary dysfunction. Electron microscopy analysis revealed ultrastructural irregularities of the dynein arms and misalignments of the outer-doublet microtubules on the ciliary axonemes, suggesting instability of the ciliary architecture in lrrc50(hu255H) mutants. TheSDS22-like leucine-rich repeats present in Lrrc50 are necessary for proper protein function, since injection of a deletion construct of the first LRR did not rescue the zebrafish mutant phenotype. Subcellular distribution of human LRRC50-EGFP in MDCK and HEK293T cells is diffusely cytoplasmic and concentrated at the mitotic spindle poles and cilium. LRRC50 RNAi knock-down in human proximal tubule HK-2 cells thoroughly recapitulated the zebrafish brush border and cilia phenotype, suggesting conservation of LRRC50 function between both species. In summary, we present the first genetic vertebrate model for lrrc50 function and propose LRRC50 to be a novel candidate gene for human cystic kidney disease, involved in regulation of microtubule-based cilia and actin-based brush border microvilli.
Figures
Figure 1.
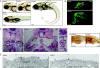
lrrc50 is involved in the development of cystic kidney disease and brush border abnormalities in zebrafish. (A) lrrc50hu255H mutants are characterized by a curved body axis, the development of pronephric tubular (PT) cysts, and dilated pronephric ducts (PD), as shown at 54 hpf and 7 dpf. (B) Confocal imaging with Cy3-labeled 3G8 (green), staining the lateral part of the PT and the anterior half of the PD in 6.5 dpf embryos. (C) Cross-sections (hematoxylin and eosin) of a wild-type and lrrc50hu255H−/− pronephros at 6.5 dpf. Mutants show a grossly distended cyst in place of the pronephric tubule (black arrow), glomerular defects, and dilated pronephric ducts. An enlargement of the glomerulus of a wild-type fish (detail insert I) and a lrrc50hu255H mutant glomerulus with dilated cells (black arrowheads; detail insert II) are depicted in the right panels. (D) BrdU incorporation demonstrates increased proliferation in the pronephric tubule and pronephric duct in lrrc50hu255H mutants at 6.5 dpf. (E) Ultrastructural analysis reveals a severe reduction of the brush border (vertical arrows) in mutant posterior pronephric duct cells at 4 dpf. A, anterior; P, posterior.
Figure 2.
lrrc50 is required for cilia function in zebrafish. (A) Dysfunctional cilia in KV are implicated in left-right polarity defects, characterized by random heart jogging in lrrc50 mutants. Absolute numbers of embryos scored are depicted in the bars; the bottom panel displays representative frontal images of the different heart jogging phenotypes. (B) Confocal analysis of cilia in the anterior (left) and posterior pronephric duct (right) immunostained with anti-acetylated α-tubulin (green) and DAPI (blue). Cilia in lrrc50hu255H mutants appear morphologically disorganized at 52 hpf. *Motor axons, which also stain for acetylated tubulin. (C) Scanning electron micrographs of the lateral line organs (LLO) show a reduction in kinocilia number and length in 7 dpf lrrc50hu255H mutants. (D) For investigation of the fluid excretion via the pronephros, 4.5 dpf embryos were administered an injection of TAMRA. Whereas in wild-type siblings the first TAMRA excretion via the cloaca (*) can be observed within 5 min, in mutants, time to excretion is markedly slower (mutant 1) or completely absent (mutant 2). White arrowheads indicate final position TAMRA. Glomerular filtration does not seem to be affected, since TAMRA dye accumulation can be seen in both the pronephric tubule and anterior pronephric duct. T, time after injection.
Figure 3.
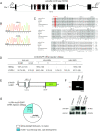
Genomic organization and expression pattern of zebrafish lrrc50. (A) Genomic organization of zebrafish lrrc50. lrrc50hu255H was mapped to chromosome 7, flanked by single-sequence length polymorphism markers z15270 (39 cM) and z8693 (41.5 cM) on the MGH mapping panel (
). Exons are represented as black boxes. Sequencing revealed a mutation in exon 4 of lrrc50, highlighted in red. (B) In lrrc50hu255H mutants, a T/A mutation is introduced, changing a conserved leucine into a premature stop codon. (C) ClustalW alignment shows Lrrc50 to be a highly conserved protein from Chlamydomonas to human (for accession numbers, see Supplemental Figure 5). LRRs are outlined and lrrc50hu255H L88X is highlighted in red. (D) The mutant phenotype is rescued by injection of 320 pg lrrc50-EGFP mRNA, as scored for body curvature, cilia motility, and pronephric cyst formation after 3 dpf. Absolute numbers of animals scored are in parentheses. (E) Schematic representation of the ΔLRR1-lrrc50-EGFP deletion construct in which the consensus LxxLxL sequence (nucleotides 391 to 408) of the first LRR is removed in frame. (F) _Hin_dIII digestion on plasmid DNA shows a reduced band in the deletion construct as compared with full-length lrrc50-EGFP. (G) ΔLRR1-lrrc50-EGFP mRNA is unable to rescue the mutant phenotype; the Mendelian sibling-to-mutant ratio is maintained. (H) Western blot analysis on _ΔLRR1-lrrc50-EGFP_–injected embryos with α-GFP shows that despite robust expression, the ΔLRR1-lrrc50-EGFP deletion construct cannot rescue the lrrc50hu255H phenotype.
Figure 4.
Zebrafish lrrc50 is expressed in all ciliated tissues. Whole-mount in situ hybridization displays expression at 90% epiboly in the dorsal forerunner cells, which aggregate to form the ciliated KV (A); 12 somites (14 hpf) in the otic placode, pronephric duct primordia, and floor plate (B); 20 hpf in the pronephric duct, neural tube, nose, and diffusely in the brain (C); and 72 hpf in nose and lateral line organs (D).
Figure 5.
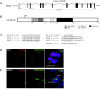
Genomic organization and subcellular localization of human LRRC50. (A) Genomic organization of human LRRC50. A putative alternative translation start site at methionine 88 is in exon 3 (ATG2). (B) Schematic representation of human LRRC50 showing the locations of six putative N-terminal LRRs, an LRRcap partially overlapping with the sixth LRR, a coiled coil, and a nonconserved proline-rich domain. Residue numbers below the bar mark putative domain boundaries. (C) hsLRR 1 to 3 show close homology to the LRR_SDS22-like consensus motif, whereas hsLRR 4 to 6 contain only the conserved consensus LRR sequence. (D) Subcellular localization of LRRC50 was determined by transfection of 293T cells with a full-length LRRC50-EGFP construct. Distribution of LRRC50-EGFP (green) appears diffusely cytoplasmic with bright spots at the spindle poles of mitotic cells as shown by co-staining with γ-tubulin (red). (E) LRRC50-EGFP was observed to localize to the ciliary structure extending from the basal body in MDCK cells but never to the basal body itself as determined by co-staining with α-γ-tubulin. Nuclei are counterstained with DAPI (blue).
Figure 6.
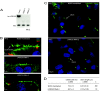
LRRC50 is required for brush border and cilia regulation in human proximal tubule cells. (A) Reverse transcriptase–PCR analysis of human LRRC50 in HK-2 cells, either mock (empty vector) transfected or with the indicated RNAi construct, demonstrating complete knockdown of LRRC50 mRNA levels. 18S RNA was taken as loading control. (B) LRRC50 RNAi knockdown with two different constructs in human proximal tubule HK-2 cells reduces the ezrin stained brush border (green). Immunostaining with zona occulans-1 (ZO-1; red) indicates intact tight junctions. (C and D) LRRC50 RNAi-2 knockdown in HK-2 cells reduces the number of ciliated cells and cilia length as shown by anti-acetylated α-tubulin staining (green). Nuclei are counterstained with DAPI (blue).
Figure 7.
Aberrant ciliary ultrastructure in lrrc50 mutant zebrafish. (A) Electron microscopy reveals ultrastructural irregularities in lrrc50hu255H −/−. Most mutant cilia lack the ODA normally present on the outer-doublet microtubules of ciliary axonemes (I); however, misplacement of the IDA (II/III) or ODA (IV) or complete lack of all dynein arms (V) is also observed. (B) Outer-doublet alignments were measured as depicted in the schematic overview. Briefly, a best-fitting ellipse was drawn through the center (red dot 2) of the doublets. Then, a straight line (a) was drawn through the middle points of the individual doublet microtubules (dots 1 to 3). The angle between the drawn tangent line (b) through dot 2 with the best fitting ellipse and line (a) was measured. (C) lrrc50hu255H mutants show a marked increase in outer-doublet misalignments.
Similar articles
- Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning.
Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. Serluca FC, et al. Development. 2009 May;136(10):1621-31. doi: 10.1242/dev.020735. Development. 2009. PMID: 19395640 Free PMC article. - Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants.
Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter CL, Serluca FC, Thiberge SY, Burdine RD. Sullivan-Brown J, et al. Dev Biol. 2008 Feb 15;314(2):261-75. doi: 10.1016/j.ydbio.2007.11.025. Epub 2007 Dec 3. Dev Biol. 2008. PMID: 18178183 Free PMC article. - The coiled-coil domain containing protein CCDC151 is required for the function of IFT-dependent motile cilia in animals.
Jerber J, Baas D, Soulavie F, Chhin B, Cortier E, Vesque C, Thomas J, Durand B. Jerber J, et al. Hum Mol Genet. 2014 Feb 1;23(3):563-77. doi: 10.1093/hmg/ddt445. Epub 2013 Sep 18. Hum Mol Genet. 2014. PMID: 24067530 - Motor or sensor: a new aspect of primary cilia function.
Yokoyama T. Yokoyama T. Anat Sci Int. 2004 Jun;79(2):47-54. doi: 10.1111/j.1447-073x.2004.00072.x. Anat Sci Int. 2004. PMID: 15218623 Review. - Ciliary Proteins: Filling the Gaps. Recent Advances in Deciphering the Protein Composition of Motile Ciliary Complexes.
Osinka A, Poprzeczko M, Zielinska MM, Fabczak H, Joachimiak E, Wloga D. Osinka A, et al. Cells. 2019 Jul 17;8(7):730. doi: 10.3390/cells8070730. Cells. 2019. PMID: 31319499 Free PMC article. Review.
Cited by
- Cilia in the developing zebrafish ear.
Whitfield TT. Whitfield TT. Philos Trans R Soc Lond B Biol Sci. 2020 Feb 17;375(1792):20190163. doi: 10.1098/rstb.2019.0163. Epub 2019 Dec 30. Philos Trans R Soc Lond B Biol Sci. 2020. PMID: 31884918 Free PMC article. Review. - Identification of testicular cancer driver genes by a cross-species comparative oncology approach.
Sanchez A, Xu L, Pierce JL, Lafin JT, Abe D, Bagrodia A, Frazier AL, Amatruda JF. Sanchez A, et al. Andrology. 2019 Jul;7(4):545-554. doi: 10.1111/andr.12644. Epub 2019 May 13. Andrology. 2019. PMID: 31087453 Free PMC article. - Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms.
Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, Wolf WE, Carson JL, Hazucha MJ, Yin W, Davis SD, Dell SD, Ferkol TW, Sagel SD, Olivier KN, Jahnke C, Olbrich H, Werner C, Raidt J, Wallmeier J, Pennekamp P, Dougherty GW, Hjeij R, Gee HY, Otto EA, Halbritter J, Chaki M, Diaz KA, Braun DA, Porath JD, Schueler M, Baktai G, Griese M, Turner EH, Lewis AP, Bamshad MJ, Nickerson DA, Hildebrandt F, Shendure J, Omran H, Zariwala MA. Knowles MR, et al. Am J Hum Genet. 2013 Oct 3;93(4):711-20. doi: 10.1016/j.ajhg.2013.07.025. Epub 2013 Sep 19. Am J Hum Genet. 2013. PMID: 24055112 Free PMC article. - Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning.
Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. Serluca FC, et al. Development. 2009 May;136(10):1621-31. doi: 10.1242/dev.020735. Development. 2009. PMID: 19395640 Free PMC article. - Zebrafish assays of ciliopathies.
Zaghloul NA, Katsanis N. Zaghloul NA, et al. Methods Cell Biol. 2011;105:257-72. doi: 10.1016/B978-0-12-381320-6.00011-4. Methods Cell Biol. 2011. PMID: 21951534 Free PMC article.
References
- Ibanez-Tallon I, Heintz N, Omran H: To beat or not to beat: Roles of cilia in development and disease. Hum Mol Genet 12: R27–R35, 2003 - PubMed
- Brokaw CJ: Flagellar movement: A sliding filament model. Science 178: 455–462, 1972 - PubMed
- Dutcher SK: Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet 11: 398–404, 1995 - PubMed
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK: Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552, 2004 - PubMed
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous