Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study - PubMed (original) (raw)
. 2008 Jun 15;111(12):5477-85.
doi: 10.1182/blood-2008-01-132837. Epub 2008 Apr 3.
Meenakshi Devidas, Stephen P Hunger, W Paul Bowman, Andrew J Carroll, William L Carroll, Stephen Linda, Paul L Martin, D Jeanette Pullen, David Viswanatha, Cheryl L Willman, Naomi Winick, Bruce M Camitta; Children's Oncology Group
Affiliations
- PMID: 18388178
- PMCID: PMC2424148
- DOI: 10.1182/blood-2008-01-132837
Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study
Michael J Borowitz et al. Blood. 2008.
Abstract
Minimal residual disease (MRD) is an important predictor of relapse in acute lymphoblastic leukemia (ALL), but its relationship to other prognostic variables has not been fully assessed. The Children's Oncology Group studied the prognostic impact of MRD measured by flow cytometry in the peripheral blood at day 8, and in end-induction (day 29) and end-consolidation marrows in 2143 children with precursor B-cell ALL (B-ALL). The presence of MRD in day-8 blood and day-29 marrow MRD was associated with shorter event-free survival (EFS) in all risk groups; even patients with 0.01% to 0.1% day-29 MRD had poor outcome compared with patients negative for MRD patients (59% +/- 5% vs 88% +/- 1% 5-year EFS). Presence of good prognostic markers TEL-AML1 or trisomies of chromosomes 4 and 10 still provided additional prognostic information, but not in National Cancer Institute high-risk (NCI HR) patients who were MRD(+). The few patients with detectable MRD at end of consolidation fared especially poorly, with only a 43% plus or minus 7% 5-year EFS. Day-29 marrow MRD was the most important prognostic variable in multi-variate analysis. The 12% of patients with all favorable risk factors, including NCI risk group, genetics, and absence of days 8 and 29 MRD, had a 97% plus or minus 1% 5-year EFS with nonintensive therapy. These studies are registered at www.clinicaltrials.gov as NCT00005585, NCT00005596, and NCT00005603.
Figures
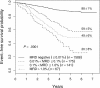
Figure 1
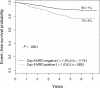
EFS of all patients enrolled on 9900 series therapeutic studies with satisfactory end-induction MRD. The 5-year EFS values plus or minus SE are shown for patients with varying levels of MRD. The outcome of those with high levels of MRD is very poor, but even those with 0.01% to 0.1% MRD have only a 59% plus or minus 5% 5-year EFS.
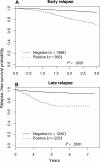
Figure 2
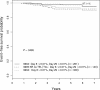
Relapse-free survival showing the effect of end-induction MRD on early and late relapse. (A) Early relapse. (B) Late relapse. MRD positivity is defined as greater than .01%. (A) All patients were censored at 3 years from diagnosis. In addition, all nonrelapse events occurring during the first 3 years were censored. (B) Only patients who were in remission at 3 years from diagnosis are included in the analysis and again, all nonrelapse events are censored.
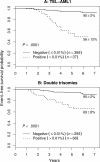
Figure 3
EFS of NCI SR patients with favorable genetic features. (A) TEL-AML1. (B) Double trisomies. The very few patients with both lesions are included in panel A as a function of end-induction MRD. Outcome of MRD+ patients in both groups is much worse than those who are MRD−. The 5-year EFS is indicated on each curve as appropriate.
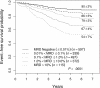
Figure 4
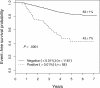
EFS of all patients enrolled in therapeutic studies as a function of level of day-8 PB MRD. There is a stepwise decrement in 5-year EFS at each 10-fold increase in MRD level.
Figure 5
Prognostic significance of day-8 blood MRD in patients who are free of MRD in bone marrow by day 29. Patients with high levels (defined as > 1%) MRD at day 8 fare worse (5-year EFS of 79% ± 4%) than those with lower levels (90% ± 1%), even if they become MRD− by day 29. This difference was especially important in NCI HR patients (see “Results”).
Figure 6
EFS among variably defined groups of good-prognosis patients. NCI SR patients with favorable genetic features who were MRD− at both day 8 and day 29 were the best group, with a 97% plus or minus 1% 5-year EFS. They had statistically better outcomes than either patients without the genetic features who had the same MRD characteristics (92% ± 3%; P = .020) or end-induction MRD−, favorable genetic patients who were day-8 MRD+ (93% ± 2%; P = .024).
Figure 7
EFS of all patients as a function of level of end-consolidation MRD. Patients who were MRD+ (> .01%) had a significantly inferior outcome, with a 5-year EFS of 43% plus or minus 7%. This effect was seen on each of the 3 therapeutic studies (see text). Prognostic significance of end-consolidation MRD in all patients enrolled on therapeutic studies.
Similar articles
- Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: A Clinical Evidence Review.
Health Quality Ontario. Health Quality Ontario. Ont Health Technol Assess Ser. 2016 Mar 8;16(7):1-52. eCollection 2016. Ont Health Technol Assess Ser. 2016. PMID: 27099643 Free PMC article. Review. - Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children's Oncology Group study.
Borowitz MJ, Pullen DJ, Shuster JJ, Viswanatha D, Montgomery K, Willman CL, Camitta B; Children's Oncology Group study. Borowitz MJ, et al. Leukemia. 2003 Aug;17(8):1566-72. doi: 10.1038/sj.leu.2403001. Leukemia. 2003. PMID: 12886244 - Persistence of TEL-AML1 fusion gene as minimal residual disease has no additive prognostic value in CD 10 positive B-acute lymphoblastic leukemia: a FISH study.
Mosad E, Hamed HB, Bakry RM, Ezz-Eldin AM, Khalifa NM. Mosad E, et al. J Hematol Oncol. 2008 Oct 17;1:17. doi: 10.1186/1756-8722-1-17. J Hematol Oncol. 2008. PMID: 18928518 Free PMC article. - Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232.
Borowitz MJ, Wood BL, Devidas M, Loh ML, Raetz EA, Salzer WL, Nachman JB, Carroll AJ, Heerema NA, Gastier-Foster JM, Willman CL, Dai Y, Winick NJ, Hunger SP, Carroll WL, Larsen E. Borowitz MJ, et al. Blood. 2015 Aug 20;126(8):964-71. doi: 10.1182/blood-2015-03-633685. Epub 2015 Jun 29. Blood. 2015. PMID: 26124497 Free PMC article. Clinical Trial. - Clinical Analysis of Pediatric T-Cell Acute Lymphoblastic Leukemia Using the MRD-Oriented Strategy System.
Xue YJ, Wang Y, Lu AD, Jia YP, Zuo YX, Ding MM, Zeng HM, Zhang LP. Xue YJ, et al. Clin Lymphoma Myeloma Leuk. 2023 Jul;23(7):477-483. doi: 10.1016/j.clml.2023.03.013. Epub 2023 Mar 29. Clin Lymphoma Myeloma Leuk. 2023. PMID: 37080879 Review.
Cited by
- Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia.
Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, Pui CH, Campana D. Faham M, et al. Blood. 2012 Dec 20;120(26):5173-80. doi: 10.1182/blood-2012-07-444042. Epub 2012 Oct 16. Blood. 2012. PMID: 23074282 Free PMC article. - Understanding the biology of CRLF2-overexpressing acute lymphoblastic leukemia.
Tasian SK, Loh ML. Tasian SK, et al. Crit Rev Oncog. 2011;16(1-2):13-24. doi: 10.1615/critrevoncog.v16.i1-2.30. Crit Rev Oncog. 2011. PMID: 22150304 Free PMC article. Review. - ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia.
Xu H, Cheng C, Devidas M, Pei D, Fan Y, Yang W, Neale G, Scheet P, Burchard EG, Torgerson DG, Eng C, Dean M, Antillon F, Winick NJ, Martin PL, Willman CL, Camitta BM, Reaman GH, Carroll WL, Loh M, Evans WE, Pui CH, Hunger SP, Relling MV, Yang JJ. Xu H, et al. J Clin Oncol. 2012 Mar 1;30(7):751-7. doi: 10.1200/JCO.2011.38.0345. Epub 2012 Jan 30. J Clin Oncol. 2012. PMID: 22291082 Free PMC article. - Minimal residual disease.
Campana D. Campana D. Leuk Suppl. 2012 Aug;1(Suppl 2):S3-4. doi: 10.1038/leusup.2012.5. Epub 2012 Aug 9. Leuk Suppl. 2012. PMID: 27175241 Free PMC article. - Minimal Residual Disease Evaluation in Childhood Acute Lymphoblastic Leukemia: A Clinical Evidence Review.
Health Quality Ontario. Health Quality Ontario. Ont Health Technol Assess Ser. 2016 Mar 8;16(7):1-52. eCollection 2016. Ont Health Technol Assess Ser. 2016. PMID: 27099643 Free PMC article. Review.
References
- Biondi A, Valsecchi MG, Seriu T, et al. Molecular detection of minimal residual disease is a strong predictive factor of relapse in childhood B-lineage acute lymphoblastic leukemia with medium risk features: a case control study of the International BFM study group. Leukemia. 2000;14:1939–1943. - PubMed
- Bjorklund E, Mazur J, Soderhall S, Porwit-MacDonald A. Flow cytometric follow-up of minimal residual disease in bone marrow gives prognostic information in children with acute lymphoblastic leukemia. Leukemia. 2003;17:138–148. - PubMed
- Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. - PubMed
- Cave H, van der Werff ten Bosch J, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia: European Organization for Research and Treatment of Cancer–Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339:591–598. - PubMed
- Ciudad J, San Miguel JF, Lopez-Berges MC, et al. Prognostic value of immunophenotypic detection of minimal residual disease in acute lymphoblastic leukemia. J Clin Oncol. 1998;16:3774–3781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources