Synthetic chemerin-derived peptides suppress inflammation through ChemR23 - PubMed (original) (raw)
Synthetic chemerin-derived peptides suppress inflammation through ChemR23
Jenna L Cash et al. J Exp Med. 2008.
Abstract
Chemerin is a chemotactic protein that binds to the G protein-coupled receptor, ChemR23. We demonstrate that murine chemerin possesses potent antiinflammatory properties that are absolutely dependent on proteolytic processing. A series of peptides was designed, and only those identical to specific C-terminal chemerin sequences exerted antiinflammatory effects at picomolar concentrations in vitro. One of these, chemerin15 (C15; A(140)-A(154)), inhibited macrophage (MPhi) activation to a similar extent as proteolyzed chemerin, but exhibited reduced activity as a MPhi chemoattractant. Intraperitoneal administration of C15 (0.32 ng/kg) to mice before zymosan challenge conferred significant protection against zymosan-induced peritonitis, suppressing neutrophil (63%) and monocyte (62%) recruitment with a concomitant reduction in proinflammatory mediator expression. Importantly, C15 was unable to ameliorate zymosan-induced peritonitis in ChemR23(-/-) mice, demonstrating that C15's antiinflammatory effects are entirely ChemR23 dependent. In addition, administration of neutralizing anti-chemerin antibody before zymosan challenge resulted in a significant exacerbation of peritoneal inflammation (up to 170%), suggesting an important endogenous antiinflammatory role for chemerin-derived species. Collectively, these results show that chemerin-derived peptides may represent a novel therapeutic strategy for the treatment of inflammatory diseases through ChemR23.
Figures
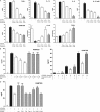
Figure 1.
Antiinflammatory activity of chemerin on activated MΦs is proteolysis dependent. (A–D) PMΦs were pretreated with 1 pM chemerin (Chem), 1 pM chemerin plus protease inhibitor (leupeptin [Leu], E-64, pefabloc [Pef], pepstatin A [Pep A], calpeptin [Cal], cathepsin S inhibitor [Cath S], cathepsin L inhibitor [Cath L]), or 1 μM dexamethasone (Dexa) for 1 h and then stimulated with 100 ng/ml LPS and 20 ng/ml IFN-γ for 15 h. Cytokine expression in MΦ supernatants after 16 h was determined by Luminex assays. mRNA levels were quantified by quantitative RT-PCR (IL-10, TGF-β) and normalized to HPRT. (B) PMΦs were pretreated with 0.1–1 pM chemerin ± 200 ng/ml PTX before LPS/IFN-γ challenge. (C) PMΦs were pretreated with 1 pM chemerin for 1 h ± PTX and then stimulated with LPS/IFN-γ for 4, 8, or 15 h. ***, P < 0.001; **, P < 0.01; *, P < 0.05 relative to LPS/IFN-γ–treated samples; ###, P < 0.001; ##, P < 0.01; #, P < 0.05 relative to chemerin-treated samples. Graphs show mean values ± SEM from three to eight independent experiments. nd, below limit of detection for this assay. ns, not significant.
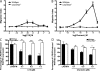
Figure 2.
Effects of chemerin and C15 peptide are impaired in MΦs from ChemR23−/− mice. (A and B) PMΦs (0.4 × 106 cells) from wild-type and ChemR23−/− mice were allowed to migrate toward chemoattractant (chemerin or C15) in the bottom well of a modified Boyden chamber over 4 h. Filters were fixed in 4% formalin, and migrated cell nuclei were stained with DAPI, visualized, and quantified. Serum-free medium was used as a negative control and 25 ng/ml RANTES (migration index, 6 ± 0.59) as a positive control. Graphs indicate mean migration index ± SEM for each treatment group (n = 4 independent experiments). ***, P < 0.001; **, P < 0.01; *, P < 0.05 relative to migration induced by the same concentration of chemoattractant in ChemR23−/− PMΦs. (C and D) PMΦs from wild-type and ChemR23−/− mice were pretreated with 0.1–1 pM C15 (C) or 0.1–1 pM chemerin (D) for 1 h and then stimulated with 100 ng/ml LPS and 20 ng/ml IFN-γ for 15 h. Mean expression of cytokines ± SEM in MΦ supernatants after 16 h was determined by ELISA (n = 4 independent experiments). **, P < 0.01; *, P < 0.05 relative to LPS/IFN-γ–treated samples. ns, not significant.
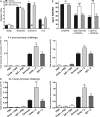
Figure 3.
C15 ameliorates zymosan-induced peritonitis in a ChemR23-dependent manner. C57Bl6/J mice were dosed i.p. with PBS or 0.32 ng/kg C15 followed by injection with PBS or 10 μg zymosan (∼2 × 106 particles per cavity) 1 h later. Peritoneal exudate cells (PECs) were harvested by peritoneal lavage at multiple time points (A and B; five to six mice/group) or after 4 h (C–E; 6–15 mice/group). Total PECs were quantified, and cellular composition (neutrophils vs. monocytes) was determined by FACS analysis. Cells were stained with Ly-6G-PE and 7/4-FITC. Gates were constructed around two populations, the neutrophils (7/4high, Ly-6Ghigh) and monocytes (7/4high, Ly-6Glow). (E) Representative FACS plots are shown for each treatment group at 4 h after zymosan injection. (F) Peritoneal lavage fluid was assayed for TNF-α, KC, IL-6, IL-1β, and JE by Luminex assay. (G) Mice (six to eight/treatment) were dosed i.p. with 10 μg zymosan and with 8 pg C15 or PBS either 1 h beforehand (C15 pretreatment) or 2 h later (C15 post-zymosan). Peritoneal lavage was performed 4 h after zymosan challenge. (H) Wild-type and ChemR23−/− mice on a 129SvEv background were dosed i.p. with PBS or 0.32 ng/kg C15 followed by injection with PBS or 10 μg zymosan 1 h later. PECs cells were harvested by peritoneal lavage 4 h after zymosan injection and characterized by FACS (six to eight mice/treatment). C15, chemerin15; Z, zymosan; ***, P < 0.001; **, P < 0.01 relative to zymosan-treated animals; nd, not detectable; ns, not significant.
Figure 4.
ChAb neutralizes chemerin species and exacerbates peritoneal inflammation. (A) PMΦs were used in MΦ chemotaxis assays (as detailed in Fig. 2) and allowed to migrate toward RANTES, chemerin, or C15 ± ChAb or control IgG. Graphs indicate mean migration index ± SEM (n = 4 independent experiments). ***, P < 0.001; **, P < 0.01 relative to chemoattractant. (B) PMΦs were pretreated with 1 pM C15 or 1 pM chemerin ± ChAb or control IgG for 1 h and then stimulated with 100 ng/ml LPS and 20 ng/ml IFN-γ for 15 h. Mean expression of RANTES ± SEM in MΦ supernatants after 16 h was determined by ELISA (n = 4 independent experiments). ***, P < 0.001 relative to LPS/IFN-γ–treated samples. (C and D) C57Bl6/J mice were dosed i.p. with PBS, 100 ng/mouse ChAb, or 100 ng/mouse control IgG followed by injection with PBS or 10 μg/cavity zymosan 1 h later. PECs were harvested 4 and 24 h after zymosan injection and processed as outlined in Fig. 3. Z, zymosan; ChAb, anti-chemerin antibody; **, P < 0.01 relative to zymosan-challenged mice; ns, not significant.
Similar articles
- Chemerin peptides promote phagocytosis in a ChemR23- and Syk-dependent manner.
Cash JL, Christian AR, Greaves DR. Cash JL, et al. J Immunol. 2010 May 1;184(9):5315-24. doi: 10.4049/jimmunol.0903378. Epub 2010 Apr 2. J Immunol. 2010. PMID: 20363975 Free PMC article. - The chemerin/ChemR23 system does not affect the pro-inflammatory response of mouse and human macrophages ex vivo.
Bondue B, De Henau O, Luangsay S, Devosse T, de Nadaï P, Springael JY, Parmentier M, Vosters O. Bondue B, et al. PLoS One. 2012;7(6):e40043. doi: 10.1371/journal.pone.0040043. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768214 Free PMC article. - Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model.
Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Luangsay S, et al. J Immunol. 2009 Nov 15;183(10):6489-99. doi: 10.4049/jimmunol.0901037. Epub 2009 Oct 19. J Immunol. 2009. PMID: 19841182 - Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism.
Bondue B, Wittamer V, Parmentier M. Bondue B, et al. Cytokine Growth Factor Rev. 2011 Oct-Dec;22(5-6):331-8. doi: 10.1016/j.cytogfr.2011.11.004. Epub 2011 Nov 25. Cytokine Growth Factor Rev. 2011. PMID: 22119008 Review. - Chemerin/chemR23 axis in inflammation onset and resolution.
Mariani F, Roncucci L. Mariani F, et al. Inflamm Res. 2015 Feb;64(2):85-95. doi: 10.1007/s00011-014-0792-7. Epub 2014 Dec 30. Inflamm Res. 2015. PMID: 25548799 Review.
Cited by
- Chemerin: A comprehensive review elucidating the need for cardiovascular research.
Ferland DJ, Watts SW. Ferland DJ, et al. Pharmacol Res. 2015 Sep;99:351-61. doi: 10.1016/j.phrs.2015.07.018. Epub 2015 Jul 23. Pharmacol Res. 2015. PMID: 26211950 Free PMC article. Review. - Elastase and tryptase govern TNFα-mediated production of active chemerin by adipocytes.
Parlee SD, McNeil JO, Muruganandan S, Sinal CJ, Goralski KB. Parlee SD, et al. PLoS One. 2012;7(12):e51072. doi: 10.1371/journal.pone.0051072. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227233 Free PMC article. - The Dual Role of Chemerin in Lung Diseases.
Lavis P, Bondue B, Cardozo AK. Lavis P, et al. Cells. 2024 Jan 16;13(2):171. doi: 10.3390/cells13020171. Cells. 2024. PMID: 38247862 Free PMC article. Review. - Chemerin-ChemR23 signaling in tooth development.
Ohira T, Spear D, Azimi N, Andreeva V, Yelick PC. Ohira T, et al. J Dent Res. 2012 Dec;91(12):1147-53. doi: 10.1177/0022034512464777. Epub 2012 Oct 9. J Dent Res. 2012. PMID: 23053848 Free PMC article. - Interface between hemostasis and adaptive immunity.
Qu Z, Chaikof EL. Qu Z, et al. Curr Opin Immunol. 2010 Oct;22(5):634-42. doi: 10.1016/j.coi.2010.08.017. Epub 2010 Oct 11. Curr Opin Immunol. 2010. PMID: 20932735 Free PMC article. Review.
References
- Taylor, P.R., L. Martinez-Pomares, M. Stacey, H.H. Lin, G.D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901–944. - PubMed
- Han, J., and R.J. Ulevitch. 2005. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6:1198–1205. - PubMed
- Wittamer, V., J.D. Franssen, M. Vulcano, J.F. Mirjolet, E. Le Poul, I. Migeotte, S. Brezillon, R. Tyldesley, C. Blanpain, M. Detheux, et al. 2003. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198:977–985. - PMC - PubMed
- Meder, W., M. Wendland, A. Busmann, C. Kutzleb, N. Spodsberg, H. John, R. Richter, D. Schleuder, M. Meyer, and W.G. Forssmann. 2003. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 555:495–499. - PubMed
- Zabel, B.A., S.J. Allen, P. Kulig, J.A. Allen, J. Cichy, T.M. Handel, and E.C. Butcher. 2005. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 280:34661–34666. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases