Cathepsin D--many functions of one aspartic protease - PubMed (original) (raw)
Review
Cathepsin D--many functions of one aspartic protease
Petr Benes et al. Crit Rev Oncol Hematol. 2008 Oct.
Abstract
For years, it has been held that cathepsin D (CD) is involved in rather non-specific protein degradation in a strongly acidic milieu of lysosomes. Studies with CD knock-out mice revealed that CD is not necessary for embryonal development, but it is indispensable for postnatal tissue homeostasis. Mutation that abolishes CD enzymatic activity causes neuronal ceroid lipofuscinosis (NCL) characterized by severe neurodegeneration, developmental regression, visual loss and epilepsy in both animals and humans. In the last decade, however, an increasing number of studies demonstrated that enzymatic function of CD is not restricted solely to acidic milieu of lysosomes with important consequences in regulation of apoptosis. In addition to CD enzymatic activity, it has been shown that apoptosis is also regulated by catalytically inactive mutants of CD which suggests that CD interacts with other important molecules and influences cell signaling. Moreover, procathepsin D (pCD), secreted from cancer cells, acts as a mitogen on both cancer and stromal cells and stimulates their pro-invasive and pro-metastatic properties. Numerous studies found that pCD/CD level represents an independent prognostic factor in a variety of cancers and is therefore considered to be a potential target of anti-cancer therapy. Studies dealing with functions of cathepsin D are complicated by the fact that there are several simultaneous forms of CD in a cell-pCD, intermediate enzymatically active CD and mature heavy and light chain CD. It became evident that these forms may differently regulate the above-mentioned processes. In this article, we review the possible functions of CD and its various forms in cells and organisms during physiological and pathological conditions.
Figures
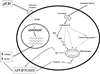
Figure 1. The role of pCD/CD in apoptosis
After the induction of apoptosis, selective permeabilization of lysosomal membrane results in the release of mature CD into cytosol. This leads to mitochondrial dysfunction. The release of CD may cause mitochondrial dysfunction either directly [114]; by ability of CD to cleave Bcl-2 family member Bid, subsequent formation of active Bax conformation and insertion of active Bax in the outer mitochondrial membrane [69]; or by interaction with unknown member of apoptotic machinery where CD enzymatic activity is not involved [124]. CD-induced mitochondrial dysfunction results in release of cyt c from mitochondria followed by activation of caspase-9 and -3 [69,114]. Alternatively, CD may trigger Bax activation via Bid-independent pathway resulting in release of apoptosis-inducing factor (AIF) and caspase-independent apoptosis [108]. In cancer cells, secreted pCD is recognized by a yet unidentified cell surface receptor. This interaction activates MAPK signaling pathway [27,188] leading to differential expression of important regulators of apoptosis [211] and may therefore affect sensitivity of cancer cells to chemotherapeutics.
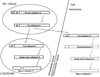
Figure 2. Forms of CD in tumors
CD is synthesized in rough endoplasmic reticulum (ER) as preprocathepsin D. In ER/Golgi pathway, signal peptide sequence is cleaved off and procathepsin D (pCD) is glycosylated at asparagine residues. Subsequently, procathepsin D is targeted to intracellular vesicular structures (lysosomes, endosomes). Upon entering these acidic compartments, the cleavage of propeptide (AP) results in a single chain intermediate enzymatically active form. Further proteolytic processing yields the mature CD which is composed of linked heavy and light chains [–4]. Enzymatically inactive pCD is secreted by cancer cells into extracellular space [25]. It was proposed that pCD can be converted in acidic extracellular milieu to enzymatically active pseudocathepsin D (form that retains residues 27–44 of the propeptide) by autocatalytic processing [17]. Pseudocathepsin D can be hypothetically processed by other proteases to single chain CD or further to mature two-chain CD. In tumors, active forms of CD can also be released from necrotic cells. The existence of particular active CD forms in extracellular space of solid tumors in vivo is therefore assumed but has never been directly evidenced.
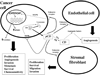
Figure 3. Proposed mechanism of pCD/CD function in cancer progression
The over-expressed pCD escapes normal targeting pathways and is secreted by the cancer cells. A small portion of secreted pCD is endocytosed via M6PR pathway, while majority of secreted pCD is recognized by a yet unidentified cell surface receptor [26,27,180,190]. This interaction activates MAPK signaling pathway [27,188] that results in differential expression of cancer promoting genes including various cytokines and NFKB2 [–213]. This, in turn, stimulates tumor growth and invasion. pCD secreted by cancer cells is also captured by stromal cells and promotes fibroblast proliferation, motility and invasion that results in cancer progression [27]. Moreover, secreted pCD may also enhance angiogenesis by stimulation of endothelial cells [215] or by enzymatic processing of angiogenic factors (after pCD → CD activation in acidic extracellular milieu) [–40].
Similar articles
- Pleiotropic effects of cathepsin D.
Vashishta A, Ohri SS, Vetvicka V. Vashishta A, et al. Endocr Metab Immune Disord Drug Targets. 2009 Dec;9(4):385-91. doi: 10.2174/187153009789839174. Endocr Metab Immune Disord Drug Targets. 2009. PMID: 19807669 Review. - Procathepsin D as a tumor marker, anti-cancer drug or screening agent.
Vetvicka V, Fusek M. Vetvicka V, et al. Anticancer Agents Med Chem. 2012 Feb;12(2):172-5. doi: 10.2174/187152012799014904. Anticancer Agents Med Chem. 2012. PMID: 22292775 Review. - The dilemma: does tissue expression of cathepsin D reflect tumor malignancy? The question: does the assay truly mirror cathepsin D mis-function in the tumor?
Nicotra G, Castino R, Follo C, Peracchio C, Valente G, Isidoro C. Nicotra G, et al. Cancer Biomark. 2010;7(1):47-64. doi: 10.3233/CBM-2010-0143. Cancer Biomark. 2010. PMID: 21045264 Review. - Possible role of procathepsin D in human cancer.
Vashishta A, Fusek M, Vetvicka V. Vashishta A, et al. Folia Microbiol (Praha). 2005;50(1):71-6. doi: 10.1007/BF02931296. Folia Microbiol (Praha). 2005. PMID: 15954536 Review. - Cathepsin D activity and selectivity in the acidic conditions of a tumor microenvironment: Utilization in the development of a novel Cathepsin D substrate for simultaneous cancer diagnosis and therapy.
Achour O, Bridiau N, Kacem M, Delatouche R, Bordenave-Juchereau S, Sannier F, Thiéry V, Piot JM, Maugard T, Arnaudin I. Achour O, et al. Biochimie. 2013 Nov;95(11):2010-7. doi: 10.1016/j.biochi.2013.07.010. Epub 2013 Jul 16. Biochimie. 2013. PMID: 23871913
Cited by
- The relationship of alpha-synuclein to mitochondrial dynamics and quality control.
Thorne NJ, Tumbarello DA. Thorne NJ, et al. Front Mol Neurosci. 2022 Aug 26;15:947191. doi: 10.3389/fnmol.2022.947191. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36090250 Free PMC article. Review. - Promotion of Endoplasmic Reticulum-Associated Degradation of Procathepsin D by Human Herpesvirus 8-Encoded Viral Interleukin-6.
Chen D, Nicholas J. Chen D, et al. J Virol. 2015 Aug;89(15):7979-90. doi: 10.1128/JVI.00375-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018151 Free PMC article. - Rapid and Progressive Loss of Multiple Retinal Cell Types in Cathepsin D-Deficient Mice-An Animal Model of CLN10 Disease.
Bassal M, Liu J, Jankowiak W, Saftig P, Bartsch U. Bassal M, et al. Cells. 2021 Mar 21;10(3):696. doi: 10.3390/cells10030696. Cells. 2021. PMID: 33800998 Free PMC article. - Coding transcriptome analyses reveal altered functions underlying immunotolerance of PEG-fused rat sciatic nerve allografts.
Smith TA, Ghergherehchi CL, Tucker HO, Bittner GD. Smith TA, et al. J Neuroinflammation. 2020 Oct 2;17(1):287. doi: 10.1186/s12974-020-01953-8. J Neuroinflammation. 2020. PMID: 33008419 Free PMC article. - A Comparative Study of Cathepsin D Expression in Peripheral and Central Giant Cell Granuloma of the Jaws by Immunohistochemistry Technique.
Zargaran M, Moghimbeigi A, Afsharmoghadam N, Nasr Isfahani M, Hashemi A. Zargaran M, et al. J Dent (Shiraz). 2016 Jun;17(2):98-104. J Dent (Shiraz). 2016. PMID: 27284554 Free PMC article.
References
- Hasilik A, Neufeld EF. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980;255:4937–4945. - PubMed
- Kornfeld S. Lysosomal enzyme targeting. Biochem Soc Trans. 1990;18:367–374. - PubMed
- Hasilik A, Neufeld EF. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980;255:4946–4950. - PubMed
- Fortenberry SC, Schorey JS, Chirgwin JM. Role of glycosylation in the expression of human procathepsin D. J Cell Sci. 1995;108:2001–2006. - PubMed
- von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous