Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis - PubMed (original) (raw)
Review
Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis
Athanasia D Panopoulos et al. Cytokine. 2008 Jun.
Abstract
Neutrophils are phagocytes whose principal function is to maintain anti-bacterial immunity. Neutrophils ingest and kill invading bacteria, releasing cytotoxic, chemotactic and inflammatory mediators at sites of infection. This serves to control the immediate host immune response and attract other cells, such as macrophages and dendritic cells, which are important for establishing long-term adaptive immunity. Neutrophils thus contribute to both the initiation and the maintenance of inflammation at sites of infection. Aberrant neutrophil activity is deleterious; suppressed responses can cause extreme susceptibility to infection while overactivation can lead to excessive inflammation and tissue damage. This review will focus on neutrophil regulation by granulocyte colony-stimulating factor (G-CSF), the principal cytokine controlling neutrophil development and function. The review will emphasize the molecular aspects of G-CSF-driven granulopoiesis in steady state (healthy) conditions and during demand-driven or 'emergency' conditions elicited by infection or clinical administration of G-CSF. Understanding the molecular control of granulopoiesis will aid in the development of new approaches designed to treat disorders of neutrophil production and function.
Figures
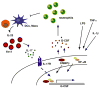
Figure 1
Schematic diagram of pathways regulating G-CSF and neutrophil production. (Right) Inflammatory stimuli in the extracellular microenvironment, such as LPS, TNFα and IL-1β act on target cells (not to scale) to induce G-CSF expression via intracellular signaling molecules such as NF-κB and C/EBPβ. (Left) IL-17 production by Th17 cells activates IL-17R signal transduction, which promotes G-CSF expression. G-CSF can be regulated by transcriptional and post-transcriptional mechanisms. (Center) The increase in G-CSF synthesis stimulates neutrophil production in the bone marrow (anatomical details are not shown). Circulating neutrophils negatively regulate the production of IL-23 and Th17 cells, providing a feedback system to control G-CSF synthesis.
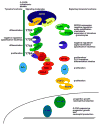
Figure 2
Functions of G-CSFR tyrosine residues and receptor-activated signaling pathways. The intracellular region of the G-CSFR is illustrated (narrow green box, left; not drawn to scale), with the four tyrosine residues highlighted in black. On the left of each tyrosine residue are functions that have been assigned by in vitro and in vivo studies, including proliferation, differentiation and macrophage/granulocyte lineage-specification. On the right of each tyrosine, representations of signaling proteins that couple to specific residues are shown. The function of these molecules is listed on the right side of the figure. At the lower portion of the figure, a schematic diagram of the nucleus of a granulocytic progenitor is shown, along with additional signaling molecules that are required for ‘emergency’ granulopoiesis. References for tyrosine and signal protein function can be found in the text.
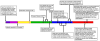
Figure 3
A timeline of major events in the development and use of G-CSF in the clinic. Left to right, the timeline highlights principal findings that are relevant to the therapeutic use of G-CSF including the initial description of congenital neutropenia [163] and leukemia in SCN [184], purification and cloning of human G-CSF [12, 15, 16], first clinical use of G-CSF for neutropenia resulting from chemotherapy or congenital origin [, , –154], cloning of G-CSFR and identification of receptor functional domains [, , –82], initial reports of G-CSFR mutations in SCN [–157], functional studies of G-CSFR mutants found in SCN [95, 96, 110, 161], and associations between the therapeutic use of G-CSF and the development of MDS/AML [9, 170, 185]. Due to space limitations, all significant references could not be included. (Timeline: Purple, 1956-1959; Pink, 1960-1969; Yellow, 1970-1979; Green, 1980-1989; Blue, 1990-1999; Red, 2000-present)
Similar articles
- STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils.
Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, Liu F, Goldsmith MA, Link DC, Murray PJ, Watowich SS. Panopoulos AD, et al. Blood. 2006 Dec 1;108(12):3682-90. doi: 10.1182/blood-2006-02-003012. Epub 2006 Aug 3. Blood. 2006. PMID: 16888100 Free PMC article. - Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization.
Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Lieschke GJ, et al. Blood. 1994 Sep 15;84(6):1737-46. Blood. 1994. PMID: 7521686 - Regulation of myeloid development and function by colony stimulating factors.
Barreda DR, Hanington PC, Belosevic M. Barreda DR, et al. Dev Comp Immunol. 2004 May 3;28(5):509-54. doi: 10.1016/j.dci.2003.09.010. Dev Comp Immunol. 2004. PMID: 15062647 Review. - Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis.
Boettcher S, Gerosa RC, Radpour R, Bauer J, Ampenberger F, Heikenwalder M, Kopf M, Manz MG. Boettcher S, et al. Blood. 2014 Aug 28;124(9):1393-403. doi: 10.1182/blood-2014-04-570762. Epub 2014 Jul 2. Blood. 2014. PMID: 24990886 Free PMC article.
Cited by
- Am80-GCSF synergizes myeloid expansion and differentiation to generate functional neutrophils that reduce neutropenia-associated infection and mortality.
Li L, Qi X, Sun W, Abdel-Azim H, Lou S, Zhu H, Prasadarao NV, Zhou A, Shimada H, Shudo K, Kim YM, Khazal S, He Q, Warburton D, Wu L. Li L, et al. EMBO Mol Med. 2016 Nov 2;8(11):1340-1359. doi: 10.15252/emmm.201606434. Print 2016 Nov. EMBO Mol Med. 2016. PMID: 27737899 Free PMC article. - Bariatric Surgery Induces Disruption in Inflammatory Signaling Pathways Mediated by Immune Cells in Adipose Tissue: A RNA-Seq Study.
Poitou C, Perret C, Mathieu F, Truong V, Blum Y, Durand H, Alili R, Chelghoum N, Pelloux V, Aron-Wisnewsky J, Torcivia A, Bouillot JL, Parks BW, Ninio E, Clément K, Tiret L. Poitou C, et al. PLoS One. 2015 May 4;10(5):e0125718. doi: 10.1371/journal.pone.0125718. eCollection 2015. PLoS One. 2015. PMID: 25938420 Free PMC article. - Inducible deletion of the Blimp-1 gene in adult epidermis causes granulocyte-dominated chronic skin inflammation in mice.
Chiang MF, Yang SY, Lin IY, Hong JB, Lin SJ, Ying HY, Chen CM, Wu SY, Liu FT, Lin KI. Chiang MF, et al. Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6476-81. doi: 10.1073/pnas.1219462110. Epub 2013 Apr 1. Proc Natl Acad Sci U S A. 2013. PMID: 23576729 Free PMC article. - STAT3 signaling in immunity.
Hillmer EJ, Zhang H, Li HS, Watowich SS. Hillmer EJ, et al. Cytokine Growth Factor Rev. 2016 Oct;31:1-15. doi: 10.1016/j.cytogfr.2016.05.001. Epub 2016 May 9. Cytokine Growth Factor Rev. 2016. PMID: 27185365 Free PMC article. Review. - Improved cytotoxic T-lymphocyte immune responses to a tumor antigen by vaccines co-expressing the SLAM-associated adaptor EAT-2.
Aldhamen YA, Seregin SS, Kousa YA, Rastall DP, Appledorn DM, Godbehere S, Schutte BC, Amalfitano A. Aldhamen YA, et al. Cancer Gene Ther. 2013 Oct;20(10):564-75. doi: 10.1038/cgt.2013.53. Epub 2013 Aug 16. Cancer Gene Ther. 2013. PMID: 23949283 Free PMC article.
References
- Huising MO, Kruiswijk CP, Flik G. Phylogeny and evolution of class-I helical cytokines. J Endocrinol. 2006;189:1–25. - PubMed
- Lutfalla G, Roest Crollius H, Stange-Thomann N, Jaillon O, Mogensen K, Monneron D. Comparative genomic analysis reveals independent expansion of a lineage-specific gene family in vertebrates: the class II cytokine receptors and their ligands in mammals and fish. BMC Genomics. 2003;4:29. - PMC - PubMed
- Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–46. - PubMed
- Zeidler C, Schwinzer B, Welte K. Congenital neutropenias. Rev Clin Exp Hematol. 2003;7:72–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009598/CA/NCI NIH HHS/United States
- T32-CA-09598-16/CA/NCI NIH HHS/United States
- R01 CA077447-03/CA/NCI NIH HHS/United States
- R01 CA077447-02/CA/NCI NIH HHS/United States
- CA77447/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous