Tlx1 and Tlx3 coordinate specification of dorsal horn pain-modulatory peptidergic neurons - PubMed (original) (raw)
Tlx1 and Tlx3 coordinate specification of dorsal horn pain-modulatory peptidergic neurons
Yi Xu et al. J Neurosci. 2008.
Abstract
The dorsal spinal cord synthesizes a variety of neuropeptides that modulate the transmission of nociceptive sensory information. Here, we used genetic fate mapping to show that Tlx3(+) spinal cord neurons and their derivatives represent a heterogeneous population of neurons, marked by partially overlapping expression of a set of neuropeptide genes, including those encoding the anti-opioid peptide cholecystokinin, pronociceptive Substance P (SP), Neurokinin B, and a late wave of somatostatin. Mutations of Tlx3 and Tlx1 result in a loss of expression of these peptide genes. Brn3a, a homeobox transcription factor, the expression of which is partly dependent on Tlx3, is required specifically for the early wave of SP expression. These studies suggest that Tlx1 and Tlx3 operate high in the regulatory hierarchy that coordinates specification of dorsal horn pain-modulatory peptidergic neurons.
Figures
Figure 1.
Persistent and transient Tlx3 expression revealed by fate mapping. A, Tau-nLacZ reporter mice were crossed with Tlx3 Cre mice, allowing the removal of the “_STOP_” cassette, flanked with loxP sites (triangles), by Cre-mediated recombination. Subsequently, the expression of nLacZ protein is driven from the pan-neuronal Tau promoter. B–E, Transverse sections through thoracic P7 spinal cord of Tau-nLacZ(Tlx3Cre) mice. B, X-gal staining marking the distribution of Tlx3+ neurons and their derivatives. C–E, Double staining of the nLacZ protein product (C–E, red) plus Pax2 (C, green), Tlx3 (D, green), or Lbx1 (E, green, arrowhead) proteins. Neurons coexpressing nLacZ and Tlx3 (D) or Lbx1 (E, arrow) appear yellow. All Tlx3+ neurons showed either strong or weak expression of nLacZ (D) (data not shown).
Figure 2.
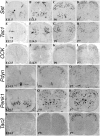
Expression of peptide precursor genes in the developing spinal cord. A–U, In situ hybridization was performed on sections through thoracic spinal cord at various developmental stages using various peptide genes as the probes. A–D, At E11.5, Sst expression was initiated in the dorsal spinal cord (A, arrow) but was already well established in the ventral spinal cord (A, arrowhead). By E13.5, a patch of _Sst_-expressing cells emerged in areas lateral to the dorsal midline (B, arrow). By P0, numerous _Sst_-expressing cells were present throughout the dorsal horn (C), and by P7, Sst expression was enriched in the superficial laminas (D). E–H, At E12.5, Tac1 expression was observed in the intermediate spinal cord area (E, arrow) and in the ventral horn at forelimb levels (E, arrowhead). By E14.5, Tac1 expression was greatly expanded (F). From P0 to P7, most _Tac1_-expressing cells still occupied deep laminas, with the remaining few scattering through superficial laminas (G, H). I–L, _CCK_-expressing cells first emerged at E14.5 in intermediate dorsal horn laminas, and the expression persists at P0–P7. M–R, _Pdyn_-expressing and _Penk1_-expressing cells emerged in the ventral spinal cord at E14.5 (M, P) and then expanded to the dorsal horn from E16.5 to P0 (N, Q) (data not shown). From P0 and on, _Pdyn_-expressing cells were enriched in superficial laminas (N, O), and _Penk1_-expressing cells were scattered throughout the spinal cord (Q, R). S–U, _Tac2_-expressing neurons emerged from P5 to P7 in an intermediate layer.
Figure 3.
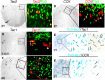
Expression of Tac2, Tac1, and CCK in Tlx3+ neurons or their derivatives. A–D, Double staining of Tlx3 protein (green) with Tac2, Tac1, or CCK mRNAs (red) on thoracic spinal sections at indicated stages. Note the consistent coexpression of Tac2 with Tlx3 (A), whereas Tac1 and CCK were expressed in both Tlx3+ (arrows) and Tlx3− neurons (arrowheads). Only cells with clear nuclear morphology were analyzed. E, F, A combination of lacZ staining and in situ hybridization. Thoracic spinal sections of P7 Tau-nLacZ(Tlx3Cre) mice were first subjected to lacZ staining (blue). After photographing, the sections were then subjected to in situ hybridization (purple staining). Note a coexpression of lacZ with Tac1 (E, arrows) or CCK (F, arrows).
Figure 4.
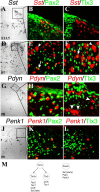
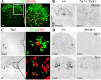
Expression of Sst, Pdyn, and Penk1 in Pax2+ or Tlx3+ neurons. A–L, Double staining of Pax2 protein (B, E, H, K, green) or Tlx3 protein (C, F, I, L, green) with Sst (A–F, red), Pdyn (G–I, red), or Penk1 (J–L, red) mRNA on spinal sections at indicated stages. Bright-field in situ hybridization signals were converted into red pseudo-fluorescent signals. A–C, Note a colocalization of Sst with Pax2 (B) but not with Tlx3 (C) in E13.5 lumbar dorsal horn. D–F, In P7 dorsal horn, Pax2 was expressed in deep lamina _Sst_-expressing neurons (E, arrow) but not in superficial _Sst_-expressing neurons (E, arrowhead). At this stage, a portion of _Sst_-expressing neurons in superficial laminas coexpressed Tlx3 (arrow). Fate mapping showed that the remaining Tlx3-negative _Sst_-expressing neurons in superficial laminas (F, arrowhead) were derived primarily from Tlx3+ neurons (supplemental Fig. 3, available at
as supplemental material). G–L, Colocalization of Pdyn and Penk1 with Pax2 (H, K) but not Tlx3 (I, L). Arrows in I indicate _Pdyn_-expressing cells with clear nuclear morphology that lack Tlx3 expression. M, Schematics show two distinct set of peptides that are associated with Tlx3+ neurons (and their derivatives) and Pax2+ neurons, respectively.
Figure 5.
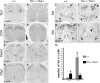
Loss of peptide gene expression in _Tlx1/3_−/− mice. A–L, In situ hybridization was performed on sections through wild-type or _Tlx1/3_−/− thoracic spinal cords at E14.5 or E18.75. A–D, Note a loss of Tac1 and CCK expression in mutants. E–H, Penk1 and Pdyn expression was not reduced (as indicated by quantitative data; see Results). I, J, Sst expression was expanded in the deep dorsal horn of E14.5 _Tlx1/3_−/− embryos (arrows). However, no Sst expression was detected in the most superficial dorsal horn (J, arrowhead). K, L, Sst expression was lost in the superficial dorsal horn of E18.75 _Tlx1/3_−/− mice (arrow), whereas expression in deep dorsal horn laminas persisted (L, arrowhead). M, Quantitative data showed the numbers of _Sst_-expressing neurons per wild-type or _Tlx1/3_−/− dorsal spinal cord section.
Figure 6.
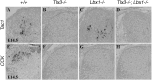
Lbx1 is required for CCK expression. A–H, In situ hybridization was performed on sections of E14.5 lumbar spinal cords with indicated genotypes, with Tac1 and CCK as the probes.
Figure 7.
Brn3a controls an early wave of Tac1 expression. A, Double staining of Tlx3 (green) and Brn3a (red, arrowhead) protein in P0 thoracic spinal cord. Coexpressing cells appear in yellow (arrow). B, In situ hybridization with the Brn3a probe on sections of the thoracic spinal cords of E18.75 wild-type and _Tlx1/3_−/− mice. Note a reduction of Brn3a expression in the mutant spinal cord. C, Double staining of Brn3a protein (green) and Tac1 mRNA (red) on sections of lumbar E12.5 and P0 wild-type spinal cords. Note that Tac1 was expressed exclusively in Brn3a+ neurons at E12.5 but in both Brn3a+ (arrow) and Brn3a− (arrowhead) cells at P0. D, In situ hybridization of Tac1 on sections through lumbar wild-type or _Brn3a_−/− spinal cords at indicated developmental stages.
Figure 8.
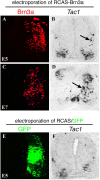
Brn3a induces Tac1 expression in the chick neural tube. A–D, Electroporation of RCAS-Brn3a (plus pCAX-GFP, not shown) into the right side of chick neural tubes at E2, followed by analysis of the expression of exogenous Brn3a protein by immunostaining (A, C) and Tac1 expression by in situ hybridization at E5 (A, B) or E7 (C, D). Arrows indicate ectopic Tac1 expression. E, F, Electroporation with control vectors (RCAS plus pCAX-GFP, referred to as RCAS/GFP). GFP expression (E) was used to monitor electroporation efficacy. Note a lack of SP induction (F).
Figure 9.
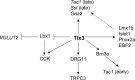
Tlx1/3 coordinate dorsal horn neuron development. Tlx3 acts to antagonize Lbx1 to control VGLUT2 expression and the glutamatergic transmitter phenotype. Both Tlx3 and Lbx1 are required for CCK expression, and transient Tlx3 expression may allow Lbx1 to work together with a putative _Tlx3_-dependent downstream event to control CCK expression (see Discussion). Tlx3 acts via Brn3a to control the early wave of Tac1 expression, but Brn3a is not required for the expression of other _Tlx3_-dependent genes such as CCK, Sst, TRPC3, or Gria2. Tlx3 acts via DRG11 to control the expression of TRPC3 (Li et al., 2006), but DRG11 is not required for the expression of any _Tlx3_-dependent peptide genes or Gria2 (C. Lopes and D. Lima, unpublished data). Tlx3 is required for the expression of many other dorsal horn transcription factors such as Islet1, Phox2a, EBF2 [wrongly called EBF3 in our previous paper (Qian et al., 2002)], and Lmx1b (Qian et al., 2002). These downstream factors may control other unknown downstream events or control the expression of those _Tlx3_-dependent but _Brn3a/DRG11_-independent target genes such as late-wave Tac1, late-wave Sst, or Gria2 (dashed arrow).
Similar articles
- Tlx1/3 and Ptf1a control the expression of distinct sets of transmitter and peptide receptor genes in the developing dorsal spinal cord.
Guo Z, Zhao C, Huang M, Huang T, Fan M, Xie Z, Chen Y, Zhao X, Xia G, Geng J, Cheng L. Guo Z, et al. J Neurosci. 2012 Jun 20;32(25):8509-20. doi: 10.1523/JNEUROSCI.6301-11.2012. J Neurosci. 2012. PMID: 22723691 Free PMC article. - Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates.
Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Cheng L, et al. Nat Neurosci. 2004 May;7(5):510-7. doi: 10.1038/nn1221. Epub 2004 Apr 4. Nat Neurosci. 2004. PMID: 15064766 - Brn3a/Pou4f1 regulates dorsal root ganglion sensory neuron specification and axonal projection into the spinal cord.
Zou M, Li S, Klein WH, Xiang M. Zou M, et al. Dev Biol. 2012 Apr 15;364(2):114-27. doi: 10.1016/j.ydbio.2012.01.021. Epub 2012 Feb 3. Dev Biol. 2012. PMID: 22326227 Free PMC article. - [Transmission and modulation of nociceptive information in the spinal dorsal horn].
Satoh M. Satoh M. Nihon Yakurigaku Zasshi. 1993 May;101(5):289-98. doi: 10.1254/fpj.101.5_289. Nihon Yakurigaku Zasshi. 1993. PMID: 8392482 Review. Japanese. - Interaction of neuropeptides and excitatory amino acids in the rat superficial spinal dorsal horn.
Randić M, Kolaj M, Kojić L, Cerne R, Cheng G, Wang RA. Randić M, et al. Prog Brain Res. 1995;104:225-53. doi: 10.1016/s0079-6123(08)61793-8. Prog Brain Res. 1995. PMID: 8552771 Review. No abstract available.
Cited by
- Targeting spinal neuropeptide Y1 receptor-expressing interneurons to alleviate chronic pain and itch.
Nelson TS, Taylor BK. Nelson TS, et al. Prog Neurobiol. 2021 Jan;196:101894. doi: 10.1016/j.pneurobio.2020.101894. Epub 2020 Aug 7. Prog Neurobiol. 2021. PMID: 32777329 Free PMC article. Review. - Regulating nociceptive transmission by VGluT2-expressing spinal dorsal horn neurons.
Wang L, Chen SR, Ma H, Chen H, Hittelman WN, Pan HL. Wang L, et al. J Neurochem. 2018 Nov;147(4):526-540. doi: 10.1111/jnc.14588. Epub 2018 Oct 31. J Neurochem. 2018. PMID: 30203849 Free PMC article. - VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch.
Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, Ji RR, Lowell BB, Ma Q. Liu Y, et al. Neuron. 2010 Nov 4;68(3):543-56. doi: 10.1016/j.neuron.2010.09.008. Neuron. 2010. PMID: 21040853 Free PMC article. - Mechanical Allodynia Circuitry in the Dorsal Horn Is Defined by the Nature of the Injury.
Peirs C, Williams SG, Zhao X, Arokiaraj CM, Ferreira DW, Noh MC, Smith KM, Halder P, Corrigan KA, Gedeon JY, Lee SJ, Gatto G, Chi D, Ross SE, Goulding M, Seal RP. Peirs C, et al. Neuron. 2021 Jan 6;109(1):73-90.e7. doi: 10.1016/j.neuron.2020.10.027. Epub 2020 Nov 11. Neuron. 2021. PMID: 33181066 Free PMC article. - Tlx3 and Runx1 act in combination to coordinate the development of a cohort of nociceptors, thermoceptors, and pruriceptors.
Lopes C, Liu Z, Xu Y, Ma Q. Lopes C, et al. J Neurosci. 2012 Jul 11;32(28):9706-15. doi: 10.1523/JNEUROSCI.1109-12.2012. J Neurosci. 2012. PMID: 22787056 Free PMC article.
References
- Caspary T, Anderson KV. Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat Rev Neurosci. 2003;4:289–297. - PubMed
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. - PubMed
- Chen Z, Rebelo A, White F, Malmberg AB, Baba H, Lima D, Woolf CJ, Basbaum AI, Anderson DJ. The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron. 2001;31:59–73. - PubMed
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS047572/NS/NINDS NIH HHS/United States
- R01 NS047710/NS/NINDS NIH HHS/United States
- P01NS047572/NS/NINDS NIH HHS/United States
- R01NS47710/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials