MALS-3 regulates polarity and early neurogenesis in the developing cerebral cortex - PubMed (original) (raw)
MALS-3 regulates polarity and early neurogenesis in the developing cerebral cortex
Karpagam Srinivasan et al. Development. 2008 May.
Abstract
Apicobasal polarity plays an important role in regulating asymmetric cell divisions by neural progenitor cells (NPCs) in invertebrates, but the role of polarity in mammalian NPCs is poorly understood. Here, we characterize the function of the PDZ domain protein MALS-3 in the developing cerebral cortex. We find that MALS-3 is localized to the apical domain of NPCs. Mice lacking all three MALS genes fail to localize the polarity proteins PATJ and PALS1 apically in NPCs, whereas the formation and maintenance of adherens junctions appears normal. In the absence of MALS proteins, early NPCs progressed more slowly through the cell cycle, and their daughter cells were more likely to exit the cell cycle and differentiate into neurons. Interestingly, these effects were transient; NPCs recovered normal cell cycle properties during late neurogenesis. Experiments in which MALS-3 was targeted to the entire membrane resulted in a breakdown of apicobasal polarity, loss of adherens junctions, and a slowing of the cell cycle. Our results suggest that MALS-3 plays a role in maintaining apicobasal polarity and is required for normal neurogenesis in the developing cortex.
Figures
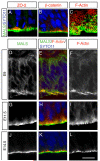
Figure 1. MALS-3 is expressed by and localizes to the apical surface of neural progenitors during neurogenesis
(A-C) Immunostaining of E14 coronal sections of rat telencephalon with antibodies against MALS-3 (green) and ZO-2, F-actin or β-catenin (red) reveal supra-apical localization of MALS-3 relative to ZO-2 (A), β-catenin (B) and F-actin (C). Nuclei are stained with SYTO-11 (blue). The en face view in panel C shows the lumen of the ventricle at the bottom, then (from bottom to top) passes through the apical-most ends of VZ cells and into the domain occupied by adherens junctions, which appear as circles of F-actin staining. (D-L) Immunostaining of coronal sections of rat telencephalon at different times during corticogenesis with antibodies against MALS (green) and ZO-2, F-actin or β-catenin (red). Nuclei are stained with SYTO-11 (blue). MALS-3 is initially localized diffusely in VZ cells (D-F), but appears to adopt an apical localization at the onset of neurogenesis (~E11.5) (G-I). This apical localization is maintained throughout the rest of embryonic life (J-L). Scale bar 5 μm.
Figure 2. Localization of MALS immunoreactivity compared with other proteins in the VZ
(A-C) Immunostaining of E14.5 coronal sections of rat telencephalon reveals that MALS is localized supra-apical to F-Actin. (D-F) CASK antibody did not reveal specific immunostaining in the VZ. (G-I) PALS1 is also localized supra-apical to F-Actin. in a pattern similar to that of MALS. (J-L) Crb3 localization is similar to that of PALS1 and MALS. (M-O) Mint shows a diffuse yet apically biased localization in the VZ relative. (P-R) Dlg, a protein localized basolaterally in many cell types, does not localize to the apical surface in NPCs. (S-U). F-Actin colocalized with staining for pan- cadherin in the apical domains of VZ cells. Scale bar 5 μm.
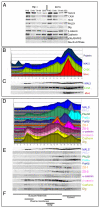
Figure 3. Distribution of proteins between membrane and cytosol in postnuclear supernatant from the embryonic telencephalon
(A) Triton soluble membranes (TX100), triton insoluble membranes (SDS) and cytosol (S100) from E14 rat telencephalon homogenate postnuclear supernatant (PNS) were prepared with either Magnesium ion (Mg+2 group) or chelating agent (EDTA group), separated by SDS-PAGE, and visualized by Western blot. The presence of either Mg+2 or EDTA resulted in no reproducible differences in molecular distributions between cytosol, Triton soluble membranes, and Triton insoluble membranes. (B, C) Integrated density plots of the distribution of MALS, CASK and Mint proteins in an iodixanol density gradient (B), with the corresponding Western blots of the different fractions (C). The distributions of MALS and CASK showed a strong similarity. (D, E) Integrated density plots of the distribution of known cell polarity proteins (D) together with corresponding western blots (E) reveal the distribution of several cell polarity proteins found in NPCs. Taken together, the data suggest that MALS might interact with CASK and PALS1 in NPCs based on their similar cellular localization. (F) Bars represent the cellular distribution of proteins known to associate with different subcellular compartments (based on data shown in Suppl. Fig. 4).
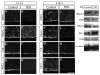
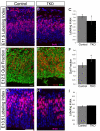
Figure 4. MALS is required for the maintenance but not establishment of apical localization of PALS1 and PATJ during corticogenesis
Coronal sections of control and MALSTKO mutant brains at E13.5 immunostained for MALS (A,B), PALS1 (C,D), Crb (E,F) and PATJ (G,H) reveal loss of MALS (B) and apical PATJ (H) relative to control. Coronal sections of control and MALSTKO mutant brains at E18.5 immunostained for aPKCζ (I, J), PALS1 (K,L), Crb (M,N) and PATJ (O,P) reveal loss of apical PALS1 (L) and PATJ (P) in MALSTKO mutants. Scale bar 10 μm. (Q) No changes were observed in total protein levels for any of the above proteins in MALSTKO mutant brains relative to control.
Figure 5. Adherens junction integrity is not compromised in MALSTKO embryos
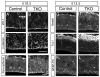
(A,B) Coronal sections of E18.5 embryos immunostained for ZO-1 to identify adherens junctions reveal normal staining in MALSTKO mutants. (C-F) Antibodies against β-catenin (C,D) and pan-cadherin (E,F), both of which localize to the apical surface at the adherens junctions, reveal no change between MALSTKO mice relative to controls. (G-L) Coronal sections of E17.5 embryos stained with antibodies against nestin (G,H) to identify neural progenitors and GFAP (K,L) to reveal glial cells reveal no change between MALSTKO mice and controls. (I,J) However, antibodies against the neuronal marker Tuj1 reveals a broader Tuj1 staining in the cortex of MALSTKO embryos, suggesting that MALSTKO progenitors quit cycling prematurely and differentiate into neurons. Scale bars 10 μm.
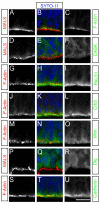
Figure 6. MALSTKO embryos display significant differences in labeling index and quit fraction during early neurogenesis
Coronal sections of E11.5 (A,B) and E12.5 (D,E) MALSTKO mutants and matching littermate control brains injected with BrdU 2 hours (A,B,G,H) or 24 hours (D,E) prior to sacrifice. Sections were immunostained with antibodies to BrdU (A,C,D,E,G,H) and Ki67 (D,E). (C) At E11.5, MALSTKO progenitors have a lower labeling index (total number of BrdU+ cells / total number of cells) relative to controls. (F) A larger number of MALSTKO mutant progenitors at E12.5 have quit the cell cycle relative to control littermates (BrdU+Ki67−/ total number of BrdU+ cells). (I) MALSTKO mutant progenitor cells recover normal cell cycle dynamics just a day later at E13.5, at which time no differences in labeling index are apparent. Scale bar 10 μm.
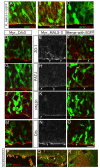
Figure 7. The mislocalization of MALS-3 disrupts polarity
Analyses of brains electroporated with FL_MALS-3/EGFP (A-C), Myr_MALS-3 (E,F,H,I,K,L,N-R) or Myr_Crb3 (D,G,J,M) at E13.5 and sacrificed at E15.5 (D-O) or E18.5 (P-R). Brains electroporated with FL-MALS-3 show no changes in the apical localization of Crb3 (A), PALS1 (B) or aPKCζ (C). Brains electroporated with Myr_Crb3 show no change in apical localization of ZO-1 (red, D), PATJ (red, G), or PALS1 (red, J) in GFP-positive cells (green). (M) Crb protein (red) is apparent in the basolateral regions of GFP-positive cells (green) following electroporation with Myr_Crb3, as expected. Myr_MALS-3 electroporated, GFP-positive cells (green) show loss of apical localization of ZO-1 (E,F), PATJ (H,I), and PALS1 (K,L). Crb staining (red) appears reduced in some GFP-positive cells (N,O). (P-Q) At E18.5, Myr_MALS-3 electroporated brains reveal misplaced cells in the lateral ventricles, breaks in the VZ, and MALS immunostaining is lost at those breaks. (P) Larger breaks and loss of PALS1 staining (red) are also observed. (Q) The ventricles are populated by delaminated cells that are Tuj1-positive (R). Scale bar 10 μm.
Similar articles
- Afadin controls cell polarization and mitotic spindle orientation in developing cortical radial glia.
Rakotomamonjy J, Brunner M, Jüschke C, Zang K, Huang EJ, Reichardt LF, Chenn A. Rakotomamonjy J, et al. Neural Dev. 2017 May 8;12(1):7. doi: 10.1186/s13064-017-0085-2. Neural Dev. 2017. PMID: 28482867 Free PMC article. - Renal defects associated with improper polarization of the CRB and DLG polarity complexes in MALS-3 knockout mice.
Olsen O, Funke L, Long JF, Fukata M, Kazuta T, Trinidad JC, Moore KA, Misawa H, Welling PA, Burlingame AL, Zhang M, Bredt DS. Olsen O, et al. J Cell Biol. 2007 Oct 8;179(1):151-64. doi: 10.1083/jcb.200702054. J Cell Biol. 2007. PMID: 17923534 Free PMC article. - Neurogenesis at the brain-cerebrospinal fluid interface.
Lehtinen MK, Walsh CA. Lehtinen MK, et al. Annu Rev Cell Dev Biol. 2011;27:653-79. doi: 10.1146/annurev-cellbio-092910-154026. Epub 2011 Jul 21. Annu Rev Cell Dev Biol. 2011. PMID: 21801012 Free PMC article. Review. - Identification of Nepro, a gene required for the maintenance of neocortex neural progenitor cells downstream of Notch.
Muroyama Y, Saito T. Muroyama Y, et al. Development. 2009 Dec;136(23):3889-93. doi: 10.1242/dev.039180. Development. 2009. PMID: 19906856 - The cell biology of neurogenesis.
Götz M, Huttner WB. Götz M, et al. Nat Rev Mol Cell Biol. 2005 Oct;6(10):777-88. doi: 10.1038/nrm1739. Nat Rev Mol Cell Biol. 2005. PMID: 16314867 Review.
Cited by
- Asymmetric segregation of the double-stranded RNA binding protein Staufen2 during mammalian neural stem cell divisions promotes lineage progression.
Kusek G, Campbell M, Doyle F, Tenenbaum SA, Kiebler M, Temple S. Kusek G, et al. Cell Stem Cell. 2012 Oct 5;11(4):505-16. doi: 10.1016/j.stem.2012.06.006. Epub 2012 Aug 16. Cell Stem Cell. 2012. PMID: 22902295 Free PMC article. - Crystallization and preliminary X-ray data collection of the L27(PATJ)-(L27N,L27C)(Pals1)-L27(MALS) tripartite complex.
Zhang J, Yang X, Shen Y, Long J. Zhang J, et al. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Nov 1;67(Pt 11):1443-7. doi: 10.1107/S174430911103689X. Epub 2011 Oct 27. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22102253 Free PMC article. - Deficiency of a membrane skeletal protein, 4.1G, results in myelin abnormalities in the peripheral nervous system.
Saitoh Y, Ohno N, Yamauchi J, Sakamoto T, Terada N. Saitoh Y, et al. Histochem Cell Biol. 2017 Dec;148(6):597-606. doi: 10.1007/s00418-017-1600-6. Epub 2017 Jul 28. Histochem Cell Biol. 2017. PMID: 28755316 - Growth and folding of the mammalian cerebral cortex: from molecules to malformations.
Sun T, Hevner RF. Sun T, et al. Nat Rev Neurosci. 2014 Apr;15(4):217-32. doi: 10.1038/nrn3707. Nat Rev Neurosci. 2014. PMID: 24646670 Free PMC article. Review. - Structure of an L27 domain heterotrimer from cell polarity complex Patj/Pals1/Mals2 reveals mutually independent L27 domain assembly mode.
Zhang J, Yang X, Wang Z, Zhou H, Xie X, Shen Y, Long J. Zhang J, et al. J Biol Chem. 2012 Mar 30;287(14):11132-40. doi: 10.1074/jbc.M111.321216. Epub 2012 Feb 15. J Biol Chem. 2012. PMID: 22337881 Free PMC article.
References
- Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–79. - PubMed
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–70. - PubMed
- Astrom KE, Webster HD. The early development of the neopallial wall and area choroidea in fetal rats. A light and electron microscopic study. Adv Anat Embryol Cell Biol. 1991;123:1–76. - PubMed
- Bohl J, Brimer N, Lyons C, Vande Pol SB. The stardust family protein MPP7 forms a tripartite complex with LIN7 and DLG1 that regulates the stability and localization of DLG1 to cell junctions. J Biol Chem. 2007;282:9392–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases