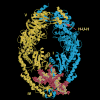DNA mismatch repair: molecular mechanism, cancer, and ageing - PubMed (original) (raw)
Review
DNA mismatch repair: molecular mechanism, cancer, and ageing
Peggy Hsieh et al. Mech Ageing Dev. 2008 Jul-Aug.
Abstract
DNA mismatch repair (MMR) proteins are ubiquitous players in a diverse array of important cellular functions. In its role in post-replication repair, MMR safeguards the genome correcting base mispairs arising as a result of replication errors. Loss of MMR results in greatly increased rates of spontaneous mutation in organisms ranging from bacteria to humans. Mutations in MMR genes cause hereditary nonpolyposis colorectal cancer, and loss of MMR is associated with a significant fraction of sporadic cancers. Given its prominence in mutation avoidance and its ability to target a range of DNA lesions, MMR has been under investigation in studies of ageing mechanisms. This review summarizes what is known about the molecular details of the MMR pathway and the role of MMR proteins in cancer susceptibility and ageing.
Figures
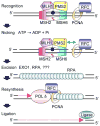
Fig. 1
Cartoon scheme for 3′-directed eukaryotic MMR. Recognition of a mismatch by MutSα (MSH2-MSH6) or MutSβ (MSH2-MSH3, not shown) and MutLα (MLH1-PMS2) results in the formation of a ternary complex whose protein-protein and protein-DNA interactions are modulated by ATP/ADP cofactors bound by MutSα and MutLα (indicated by red *). PCNA may play an important role in the recruitment of MMR proteins to the vicinity of the replication fork via a PIP motif on MSH6 and MSH3. Nicking by the endonuclease function of PMS2 stimulated by ATP, PCNA, and RFC and relevant protein-protein interactions (indicated by green arrow) may establish strand discrimination targeting repair to the newly synthesized strand. MMR is bidirectional and can be 5′-directed as well; this is not shown. HMGB1, a nonhistone chromatin protein that bends DNA also facilitates MMR in vitro at or before the excision step (not shown). Excision by EXO1 and possibly other as yet unidentified exonucleases leads to the formation of an RPA-coated single-strand gap. Resynthesis by replicative polδ and ligation restore the integrity of the duplex. See text for details.
Fig. 2
Structural model for T. aquaticus MutS bound to a mismatched DNA. The two protein monomers containing a deletion of the C-terminal 43 amino acids are shown in yellow and blue. The mismatched DNA containing a single unpaired T is shown in pink and red. Domains I and IV constitute the mismatch binding site. Two composite nucleotide binding sites reside in domain V. The H-U-H helix-u-turn-helix motif is essential for subunit dimerization.
Fig. 3
Structural model for human MutSα with HNPCC mutations. Four views of MutSα related by 90° rotations as indicated, with positions of HNPCC missense mutations indicated by spheres. Hypothetical functional classification of mutations is indicated by sphere colour (see legend). MSH2 and MSH6 are shown as light and dark grey Cα chain traces, respectively, and the DNA is coloured orange. Three clusters of surface mutations, which may correspond to sites of protein-protein interactions are indicated with dashed ovals. Reproduced with permission (Warren et al., 2007).
Similar articles
- Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis.
Sameer AS, Nissar S, Fatima K. Sameer AS, et al. Eur J Cancer Prev. 2014 Jul;23(4):246-57. doi: 10.1097/CEJ.0000000000000019. Eur J Cancer Prev. 2014. PMID: 24614649 Review. - Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics.
Lagerstedt Robinson K, Liu T, Vandrovcova J, Halvarsson B, Clendenning M, Frebourg T, Papadopoulos N, Kinzler KW, Vogelstein B, Peltomäki P, Kolodner RD, Nilbert M, Lindblom A. Lagerstedt Robinson K, et al. J Natl Cancer Inst. 2007 Feb 21;99(4):291-9. doi: 10.1093/jnci/djk051. J Natl Cancer Inst. 2007. PMID: 17312306 - Constitutive deficiency in DNA mismatch repair.
Felton KE, Gilchrist DM, Andrew SE. Felton KE, et al. Clin Genet. 2007 Jun;71(6):483-98. doi: 10.1111/j.1399-0004.2007.00803.x. Clin Genet. 2007. PMID: 17539897 Review. - Phenotype associated with recessively inherited mutations in DNA mismatch repair (MMR) genes.
de Vos M, Hayward B, Bonthron DT, Sheridan E. de Vos M, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):718-20. doi: 10.1042/BST0330718. Biochem Soc Trans. 2005. PMID: 16042583 Review. - Constitutive deficiency in DNA mismatch repair: is it time for Lynch III?
Felton KE, Gilchrist DM, Andrew SE. Felton KE, et al. Clin Genet. 2007 Jun;71(6):499-500. doi: 10.1111/j.1399-0004.2007.00801.x. Clin Genet. 2007. PMID: 17539898
Cited by
- Structural and Dynamical Properties of Nucleic Acid Hairpins Implicated in Trinucleotide Repeat Expansion Diseases.
Pan F, Xu P, Roland C, Sagui C, Weninger K. Pan F, et al. Biomolecules. 2024 Oct 10;14(10):1278. doi: 10.3390/biom14101278. Biomolecules. 2024. PMID: 39456210 Free PMC article. Review. - Localization and discrimination of GG mismatch in duplex DNA by synthetic ligand-enhanced protein nanopore analysis.
Lyu W, Zhu J, Huang X, Chinappi M, Garoli D, Gui C, Yang T, Wang J. Lyu W, et al. Nucleic Acids Res. 2024 Nov 11;52(20):12191-12200. doi: 10.1093/nar/gkae884. Nucleic Acids Res. 2024. PMID: 39413157 Free PMC article. - Mechanisms of DNA Damage Response in Mammalian Oocytes.
Sun F, Sutovsky P, Patterson AL, Balboula AZ. Sun F, et al. Adv Anat Embryol Cell Biol. 2024;238:47-68. doi: 10.1007/978-3-031-55163-5_3. Adv Anat Embryol Cell Biol. 2024. PMID: 39030354 Review. - Genomic and Epigenomic Biomarkers of Immune Checkpoint Immunotherapy Response in Melanoma: Current and Future Perspectives.
Hossain SM, Carpenter C, Eccles MR. Hossain SM, et al. Int J Mol Sci. 2024 Jun 30;25(13):7252. doi: 10.3390/ijms25137252. Int J Mol Sci. 2024. PMID: 39000359 Free PMC article. Review. - Predictive modeling of gene mutations for the survival outcomes of epithelial ovarian cancer patients.
Ma MC, Lavi ES, Altwerger G, Lin ZP, Ratner ES. Ma MC, et al. PLoS One. 2024 Jul 8;19(7):e0305273. doi: 10.1371/journal.pone.0305273. eCollection 2024. PLoS One. 2024. PMID: 38976671 Free PMC article.
References
- Acharya S, Foster PL, Brooks PJ, Fishel R. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Molecular Cell. 2003;12:233–246. - PubMed
- An Q, Robins P, Lindahl T, Barnes DE. 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Research. 2007;67:940–945. - PubMed
- Annett K, Duggan O, Freeburn R, Hyland P, Pawelec G, Barnett Y. An investigation of DNA mismatch repair capacity under normal culture conditions and under conditions of supra-physiological challenge in human CD4+T cell clones from donors of different ages. Experimental Gerontology. 2005;40:976–981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical