Adenomatous polyposis coli plays a key role, in vivo, in coordinating assembly of the neuronal nicotinic postsynaptic complex - PubMed (original) (raw)
Adenomatous polyposis coli plays a key role, in vivo, in coordinating assembly of the neuronal nicotinic postsynaptic complex
Madelaine M Rosenberg et al. Mol Cell Neurosci. 2008 Jun.
Abstract
The neuronal nicotinic synapse plays a central role in normal cognitive and autonomic function. Molecular mechanisms that direct the assembly of this synapse remain poorly defined, however. We show here that adenomatous polyposis coli (APC) organizes a multi-molecular complex that is essential for targeting alpha3(*)nAChRs to synapses. APC interaction with microtubule plus-end binding protein EB1 is required for alpha3(*)nAChR surface membrane insertion and stabilization. APC brings together EB1, the key cytoskeletal regulators macrophin and IQGAP1, and 14-3-3 adapter protein at nicotinic synapses. 14-3-3, in turn, links the alpha3-subunit to APC. This multi-molecular APC complex stabilizes the local microtubule and F-actin cytoskeleton and links postsynaptic components to the cytoskeleton--essential functions for controlling the molecular composition and stability of synapses. This work identifies macrophin, IQGAP1 and 14-3-3 as novel nicotinic synapse components and defines a new role for APC as an in vivo coordinator of nicotinic postsynaptic assembly in vertebrate neurons.
Figures
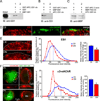
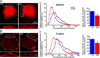
Figure 1. Selective blockade of APC::EB1 interactions led to decreases in α3*nAChR surface clusters on CG neurons i_n vivo_
(A–C) In vitro binding assays demonstrate that APC::EB1-dn selectively blocks APC binding to EB1. (A) Purified GST-APC::EB1-dn peptide specifically binds to MBP-EB1 fusion protein, while GST alone does not. (A,B) Input: 15% of MBP-EB1 total input. IB: EB1 antibody. (B,C) APC::EB1-dn peptide specifically prevented the binding of EB1 (B), but not of PSD-93 (C), to GST-APC C-terminal peptide (that contains the EB1- and PSD-93- binding domains of APC) (compare lane 1 and 2 in B and C). (C) IB: MBP antibody. (D) Confocal micrographs of immunostained acutely dissociated E11–13 CG neurons show that EB1 clusters (red) are in close proximity to and partially overlap (yellow) with α3*nAChR surface clusters (green). (E) Expression of APC::EB1-dn (DN), in vivo, causes reductions in EB1 clusters in the vicinity of the neuron surface membrane, marked by a white dashed line. Middle panel, frequency distribution graph shows reductions in the pixel intensity of EB1 labeling near the surface in APC::EB1-dn infected neurons (red boxes) versus uninfected control neurons (blue triangles) at matched ages (see below). Dashed vertical lines indicate the median intensity values. Right panel, bar graph showing 26% lower mean intensity levels for EB1 labeling near the surface in APC::EB1-dn neurons compared to control neuron values (*p<9.3×10−7, n=15 DN neurons, 11 Ctl neurons). Bars represent the mean ± SEM. In contrast, APC::EB1-dn expression caused no significant change in labeling for other APC binding partners (Supplemental Fig.1), suggesting that the blocking peptide specifically prevented the targeted interaction. (F) Epifluorescence micrographs of double-labeled E11–13 CG frozen sections showing that α3*nAChR surface clusters (red) were decreased in neurons expressing the HA-tagged APC::EB1-dn (DN, green) as compared to a control neuron (Ctl) from an uninfected CG age-matched and processed in parallel (upper right panel) and an internal control neuron in the infected CG (DN-, lower panel). HA staining (green) shows that retroviral infection was restricted to CG neurons and occasionally a few glial cells (small HA+ cells; not seen here) that surround the neuronal somata. The dark unstained region in the infected neuron cytoplasm is the nucleus. Insets, two-fold magnification views of boxed regions. Middle and right panels, α3*nAChR surface labeling shows substantial decreases in pixel intensity levels and 31% lower mean intensity levels in APC::EB1-dn neurons compared to uninfected control neuron values (*p<10−6, Student’s t-test, n=15 DN and 15 Ctl neurons). Dashed vertical lines indicate the median intensity values. Bars represent the mean ± SEM. For the quantitative assessments, the fluorescence pixel intensities were measured along 3 µm length segments of the brightest labeled surface regions (n= 2–3 segments per neuron, 10–30 DN and Ctl neurons, and 6–12 embryos for each immunolabeling experiment). The values were binned into incremental groups of 10 pixel intensity steps (from 0–9, 10–19,…, up to saturation). The percentage of pixels that belonged to each pixel intensity category was calculated and the data plotted as a relative frequency distribution (E, F).
Figure 2. APC::EB1-dn did not cause reductions in neighboring surface clusters of α7-nAChRs and glycine receptors
(A,D) Epifluorescence micrographs of double-labeled E11–13 CG frozen sections showing no decrease in surface clusters of α7-nAChRs (A, red) and GlyRs (D, red) in neurons expressing the HA-tagged APC::EB1-dn (A,D, green) as compared to uninfected control neurons. Insets, two-fold magnification views of boxed regions. (B,C) α7-nAChR surface labeling shows no significant difference in pixel intensity levels in APC::EB1-dn infected neurons versus uninfected control neurons at matched ages (p>0.14, n= 27 DN and 21 Ctl neurons). (E,F) In contrast, GlyR surface labeling shows a shift to higher pixel intensity levels (E) and an 18% increase in the mean intensity levels (F) in APC::EB1-dn neurons relative to control neuron values (* p<0.02, n= 12–13 neurons). (B,E) Dashed vertical lines indicate the median intensity values. (C,F) Bars represent the mean ± SEM.
Figure 3. Expression of a different blocking peptide, APC::PSD-93-dn, does not alter α3*nAChR surface clusters on CG neurons in vivo
(A,B) In vitro binding assays show that a different blocking peptide, APC::PSD-93-dn substantially decreased the binding of MBP-PSD-93, but not of MBP-EB1, to APC C-terminal recombinant peptide (compare lane 1 and 2 in A and B) IB: MBP antibody (A) and EB1 antibody (B). (C) Epifluorescence micrographs of immunostained E11–13 CG frozen sections showing no apparent change in α3*nAChR surface clusters (red) on HA-tagged APC::PSD-93-dn expressing neurons (green, PSD93-DN) as compared to control uninfected neurons (Ctl) age-matched and processed in parallel. Insets, two-fold magnification views of boxed regions. (D,E) α3*nAChR surface labeling shows no significant difference in pixel intensity levels in APC::PSD-93-dn infected neurons relative to uninfected control neurons (p>0.14, Students t-test; D,E, n=23 DN neurons, 30 Ctl neurons). (D) Dashed vertical lines indicate the median intensity values. (E) Bars represent the mean ± SEM.
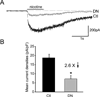
Figure 4. APC::EB1-dn expression decreases functional α3*nAChR levels
(A) Whole-cell voltage clamp recordings of α3*nAChR-mediated currents evoked by the agonist nicotine at 20µM from acutely dissociated neurons of APC::EB1-dn infected CGs (gray) and uninfected control CGs (black). Nicotine application time is indicated by the horizontal bar. (B) Bar graph showing 2.6-fold decreases in α3*nAChR mean current densities in APC::EB1-dn neurons compared to uninfected control neurons (*p<2×10−5, Student’s t-test, n= 15 DN neurons, 21 Ctl neurons). Bars represent the mean ± SEM.
Figure 5. APC::EB1-dn expression altered rates of insertion and endocytosis of surface α3*nAChRs
(A) Representative epifluorescence micrographs showing fewer newly-inserted α3*nAChRs on APC::EB1-dn (DN) neurons relative to age-matched control uninfected neurons (Ctl). Dissociated neuron cultures were first exposed to saturating amounts of anti-α3*nAChR antibody (mAb35) and unlabeled antibody Fab fragments to block existing surface α3*nAChRs, and then the appearance of newly-inserted receptor was followed over time (after 8 hours in A) using fluorescently-labeled antibody labeling (Lu et al., 2001; Man et al., 2003). (B) Graph showing four-fold decreases in the rate of α3*nAChR de novo insertion into the surface membrane on APC::EB1-dn infected neurons (white circles) as compared to uninfected control neurons (black circles) (n= 11 neurons for each time point). We quantified the number of α3*nAChR surface clusters per neuron soma and normalized to total surface area. Insertion rates were calculated from the slope of the lines. (C) Representative immunoblot showing lower levels of biotinylated surface α3*nAChRs remaining at 5 and 10 hours post-biotinylation on APC::EB1-dn infected neurons as compared to uninfected control neurons in standard cell surface biotinylation assays of endocytosis. (D) Graph of quantitative immunoblot data shows 1.6-fold faster turnover rate for surface α3*nAChRs in APC::EB1-dn infected neurons (white circles) as compared to uninfected control neurons (black circles). (E) Representative immunoblot of biotinylated surface N-cadherin, another nicotinic synapse-specific surface membrane protein. In contrast to α3*nAChRs, N-cadherin turnover rates are not altered by APC::EB1-dn expression. n= 5 separate experiments.
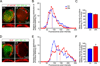
Figure 6. APC::EB1-dn expression led to decreased accumulation of the cytoskeletal regulatory proteins MACF and IQGAP1 at the neuron surface
(A,B) Confocal micrographs of double-labeled acutely dissociated E11–13 CG neurons showing that MACF (A, red) and IQGAP1 (B, red) clusters at the surface predominantly colocalized with α3*nAChR surface clusters (green; overlap indicated by yellow). Right panels, The red and green fluorescence intensity profiles co-varied for MACF and IQGAP1 with α3*nAChR surface clusters. (C, D) Confocal micrographs show decreases in MACF (C) and IQGAP1 (D) accumulation near the surface in single dissociated APC::EB1-dn neurons (DN) as compared to control uninfected neurons (Ctl). Insets, two-fold magnification views of boxed regions. Middle and right panels, MACF (C) and IQGAP1 (D) labeling near the neuron surface show shifts to lower pixel intensity levels, as well as 31% and 40% reductions in mean intensity levels in APC::EB1-dn neurons relative to control neuron values (MACF: *p<5.3×10−7, IQGAP1: *p<2.4×10−14, n= 14–19 neurons). Dashed vertical lines indicate the median intensity values. Bars represent the mean ± SEM.
Figure 7. APC::EB1-dn caused reductions in tubulin and F-actin accumulation near the neuron surface
(A,B) Confocal micrographs show decreases in accumulation near the surface membrane of neuronal-specific tubulin (A, red) and F-actin (B, red) in single dissociated APC::EB1-dn neurons (DN) as compared to control uninfected neurons (Ctl). The reductions in tubulin staining are indicative of a change in microtubule organization in APC::EB1-dn expressing neurons. Insets, two-fold magnification views of boxed regions. Middle and right panels: Tubulin (A) and F-actin (B) labeling near the surface show shifts to lower pixel intensity levels, as well as 30% and 27% decreases in mean intensity levels in APC::EB1-dn neurons relative to control neuron values (tubulin: *p<0.003, F-actin: *p<0.002, n= 10–13 neurons). Dashed vertical lines indicate the median intensity values. Bars represent the mean ± SEM.
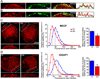
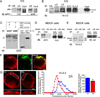
Figure 8. α3*nAChRs link to APC via 14-3-3 adaptor protein
APC (A) coimmunoprecipitated with α3-nAChRs and 14-3-3 (B) co-precipitated with α3-nAChRs and APC from CG lysates. CG homogenates were immunoprecipitated (IP) with: (A,B) α3-nAChR antibodies mAb313 (A,B) and mAb35 (B) to two different α3 subunit epitopes, (B) APC antibody C20, (A,B) HA antibody as a negative control or (B) pan- 14-3-3 antibody as a positive control. The precipitate and 1–2% of total input were separated by SDS-PAGE and immunoblotted (IB) with: (A) APC antibody C-20 or ab58 to two different epitopes or (B) pan-14-3-3 antibody that recognized two bands (~30 and ~33 KDa), as previously reported (Peng et al., 1997); APC ~250 KDa; (B) LC, IgG light chain. (C) The α3 long cytoplasmic loop fusion peptide directly binds to GST-14-3-3, but not to GST alone, in in vitro pull down assays. (D,E) APC (D) and 14-3-3 (E) co-precipitated with myc-tagged chimeric α7-nAChR subunits containing the α3 long cytoplasmic loop (α3L), but not with myc-tagged chimeric α7 containing the mutated α3 loop (S415A mutation in the 14-3-3 binding consensus motif, α3L mut) from transfected MDCK cell lysates, suggesting that 14-3-3 links the α3 long cytoplasmic loop and APC. (D,E) 2% of total lysate shows similar expression levels for α3L and α3L mut. Endogenous APC and 14-3-3 expression levels were also similar in MDCK cells transfected with the different α3-loop constructs (data not shown). n=3 or more separate experiments. (F) Epifluorescence micrographs of double-labeled E13 CG frozen sections showing that 14-3-3 (red) is enriched at synapses and co-localizes with α3*nAChR surface clusters (green; overlap, yellow). (G) Confocal micrographs show decreases in 14-3-3 accumulation near the surface in APC::EB1-dn neurons (DN) as compared to control uninfected neurons (Ctl). Insets, two-fold magnification views of boxed regions. Middle and right panels, 14-3-3 labeling near the neuron surface shows a shift to lower pixel intensity levels, and 23% lower mean intensity levels in APC::EB1-dn neurons relative to control neuron values (*p<1.3×10−8,, n= 35–42 neurons). Dashed vertical lines indicate the median intensity values. Bars represent the mean ± SEM.
Similar articles
- The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo.
Rosenberg MM, Yang F, Mohn JL, Storer EK, Jacob MH. Rosenberg MM, et al. J Neurosci. 2010 Aug 18;30(33):11073-85. doi: 10.1523/JNEUROSCI.0983-10.2010. J Neurosci. 2010. PMID: 20720115 Free PMC article. - Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein.
Temburni MK, Rosenberg MM, Pathak N, McConnell R, Jacob MH. Temburni MK, et al. J Neurosci. 2004 Jul 28;24(30):6776-84. doi: 10.1523/JNEUROSCI.1826-04.2004. J Neurosci. 2004. PMID: 15282282 Free PMC article. - Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons.
Farías GG, Vallés AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, Barrantes FJ, Inestrosa NC. Farías GG, et al. J Neurosci. 2007 May 16;27(20):5313-25. doi: 10.1523/JNEUROSCI.3934-06.2007. J Neurosci. 2007. PMID: 17507554 Free PMC article. - Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo.
Temburni MK, Blitzblau RC, Jacob MH. Temburni MK, et al. J Physiol. 2000 May 15;525 Pt 1(Pt 1):21-9. doi: 10.1111/j.1469-7793.2000.00021.x. J Physiol. 2000. PMID: 10811721 Free PMC article. Review. - An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors.
Ferns M. Ferns M. Molecules. 2021 May 21;26(11):3065. doi: 10.3390/molecules26113065. Molecules. 2021. PMID: 34063759 Free PMC article. Review.
Cited by
- Wnt signaling in neuromuscular junction development.
Koles K, Budnik V. Koles K, et al. Cold Spring Harb Perspect Biol. 2012 Jun;4(6):a008045. doi: 10.1101/cshperspect.a008045. Cold Spring Harb Perspect Biol. 2012. PMID: 22510459 Free PMC article. Review. - The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo.
Rosenberg MM, Yang F, Mohn JL, Storer EK, Jacob MH. Rosenberg MM, et al. J Neurosci. 2010 Aug 18;30(33):11073-85. doi: 10.1523/JNEUROSCI.0983-10.2010. J Neurosci. 2010. PMID: 20720115 Free PMC article. - Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain.
Takács VT, Freund TF, Nyiri G. Takács VT, et al. PLoS One. 2013 Sep 5;8(9):e72450. doi: 10.1371/journal.pone.0072450. eCollection 2013. PLoS One. 2013. PMID: 24039767 Free PMC article. - Looking below the surface of nicotinic acetylcholine receptors.
Stokes C, Treinin M, Papke RL. Stokes C, et al. Trends Pharmacol Sci. 2015 Aug;36(8):514-23. doi: 10.1016/j.tips.2015.05.002. Epub 2015 Jun 8. Trends Pharmacol Sci. 2015. PMID: 26067101 Free PMC article. Review. - Alternative splice isoforms of small conductance calcium-activated SK2 channels differ in molecular interactions and surface levels.
Scholl ES, Pirone A, Cox DH, Duncan RK, Jacob MH. Scholl ES, et al. Channels (Austin). 2014;8(1):62-75. doi: 10.4161/chan.27470. Epub 2014 Jan 6. Channels (Austin). 2014. PMID: 24394769 Free PMC article.
References
- Aitken A, Baxter H, Dubois T, Clokie S, Mackie S, Mitchell K, Peden A, Zemlickova E. Specificity of 14-3-3 isoform dimer interactions and phosphorylation. Biochem Soc Trans. 2002;30:351–360. - PubMed
- Akhmanova A, Hoogenraad CC. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol. 2005;17:47–54. - PubMed
- Brown MD, Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK34928/DK/NIDDK NIH HHS/United States
- P30 DK034928/DK/NIDDK NIH HHS/United States
- NS21725/NS/NINDS NIH HHS/United States
- R01 NS021725-23/NS/NINDS NIH HHS/United States
- P30 NS047243/NS/NINDS NIH HHS/United States
- R01 NS021725/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous