Kinetics of anti-carcinoembryonic antigen antibody internalization: effects of affinity, bivalency, and stability - PubMed (original) (raw)
Kinetics of anti-carcinoembryonic antigen antibody internalization: effects of affinity, bivalency, and stability
Michael M Schmidt et al. Cancer Immunol Immunother. 2008 Dec.
Abstract
Theoretical analyses suggest that the cellular internalization and catabolism of bound antibodies contribute significantly to poor penetration into tumors. Here we quantitatively assess the internalization of antibodies and antibody fragments against the commonly targeted antigen carcinoembryonic antigen (CEA). Although CEA is often referred to as a non-internalizing or shed antigen, anti-CEA antibodies and antibody fragments are shown to be slowly endocytosed by LS174T cells with a half-time of 10-16 h, a time scale consistent with the metabolic turnover rate of CEA in the absence of antibody. Anti-CEA single chain variable fragments (scFvs) with significant differences in affinity, stability against protease digestion, and valency exhibit similar uptake rates of bound antibody. In contrast, one anti-CEA IgG exhibits unique binding and trafficking properties with twice as many molecules bound per cell at saturation and significantly faster cellular internalization after binding. The internalization rates measured herein can be used in simple computational models to predict the microdistribution of these antibodies in tumor spheroids.
Figures
Fig. 1
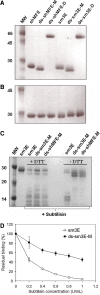
Production and characterization of anti-CEA scFvs. a, b SDS-PAGE analysis of scFvs under non-reducing (a) and reducing (b) conditions. Purified scFvs were run on a 12% Bis–Tris gel with or without 100 mM DTT. All antibody fragments run at their expected molecular weights and are ~99% pure. c Cleavage of interdomain linker confirms that interdomain disulfide bond is formed in ds-scFvs. The peptide linker connecting the V H and V L domains of each scFv was cleaved with low concentrations of subtilisin, and samples were analyzed by SDS-PAGE. Following linker cleavage, the native scFvs run as 14 kDa proteins on a non-reducing SDS-PAGE gel indicative of separate V H and V L domains, while the disulfide stabilized fragments run as 27 kDa proteins as the interdomain disulfide holds the V H and V L together. In the presence of DTT, the interdomain disulfide is reduced and the digested ds-scFvs run as separate V H and V L domains. d Interdomain disulfide bond increases functional protease stability of ds-scFvs. Alexa-488 labeled anti-CEA scFvs with or without the interdomain disulfide bond were incubated with increasing concentration of subtilisin and used to label fixed LS174T cells at subsaturating concentrations. The disulfide stabilized fragment maintains significantly greater binding activity following protease treatment
Fig. 2
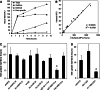
Net cellular uptake rates of anti-CEA antibodies. a Surface quenching allows for distinction of surface and internal antibody fractions. LS174T cells were continuously incubated at 37°C in the presence of Alexa-488 labeled antibodies. Total cellular fluorescence was measured at each time point by flow cytometry and the internal and surface fractions determined by surface quenching with an anti-Alexa-488 IgG as described in “Materials and methods”. Non-specific uptake was measured by pre-blocking CEA with a 100-fold excess of unlabeled antibody and was generally low. Data pictured is for an individual experiment with ds-sm3E-D. b Derivation of internalization rate k e. Internal fluorescence values measured as in a are plotted against the integral of the surface fluorescence determined by the trapezoidal rule and fit to a linear curve. The slope of the linear fit is the internalization rate k e. Data points pictured are pooled from four separate experiments with ds-sm3E-D. c Anti-CEA antibodies are generally internalized on a 10–16 h time scale. Internalization rates were determined for all antibodies and plotted as a half-time for antibody net uptake. With the exception of IgG M85151a, all tested antibodies are internalized slowly with a half time of 10–16 h. IgG M85151a is internalized significantly faster with a half-time of ~5 h (*p < 0.001). Half-times are the average of three to six individual experiments for each antibody and error bars are SD. d CEA expressing HT-1080 cells rapidly internalize ds-sm3E-M. Cellular internalization rates of Alexa-488 labeled ds-sm3E-M were determined in multiple CEA expressing cell types using the fluorescence quenching protocol described above. Colon carcinoma lines LIM1215 and SW-1222 internalize the scFv at similar rates as LS174T, while HT-1080 fibrosarcoma cells transfected with a CEA expression plasmid internalize the antibody fragment more rapidly
Fig. 3
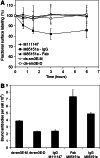
Internalization limits antibody persistence on cell surface. LS174T cells were pulse labeled with Alexa-488 conjugated antibodies on ice then chased at 37°C. At each time point, cells were surface labeled with a PE conjugated secondary antibody, and the Alexa-488 signal (total cell associated antibody) and PE signal (surface antibody) measured by flow cytometry. The difference between the total and surface antibody pools represents internalized antibodies. The high affinity scFv sm3E (a) is slowly endocytosed from the cell surface while IgG M85151a (b) displays a more rapid decrease in surface antibody levels due to faster internalization
Fig. 4
Imaging anti-CEA antibody uptake. a Anti-CEA antibodies are trafficked into intracellular pools at 37°C. Trypsinized LS174T cells were surface labeled with Alexa-488 labeled IgG M85151a on ice and incubated for 24 h at 4°C or 37°C. Cells were then labeled with goat-anti-mouse-PE and imaged on a deconvolution microscope. When incubated at 37°C a significant fraction of the 488 labeled anti-CEA antibodies are endocytosed into an intracellular pool where they are not labeled by the secondary antibody. Scale bar, 20 μm. b Internalized anti-CEA antibodies partially colocalize with markers of endocytosis. LS174T cells were incubated overnight at 37°C with fluorescently labeled anti-CEA scFvs. Cells were then washed and incubated for 1 h with fluorescently labeled markers of endocytic and lysosomal pathways. Dual label images were taken on a deconvolution microscope. The anti-CEA scFv shows partial but incomplete colocalization with all endocytic pathway markers. Each image is a clump of 5–10 adherent LS174T cells. Scale bar, 10 μm
Fig. 5
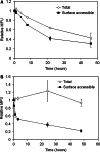
Effect of antibodies on surface CEA levels. a IgG M85151a partially downregulates surface CEA levels. LS174T cells were incubated with unlabeled antibodies at 37°C and the relative amount of surface CEA measured at each time point by labeling with an Alexa-488 labeled non-competitive antibody. Incubation with IgG M85151a decreases surface CEA ~20%, while the other antibodies have no effect on surface CEA. All measurements done in triplicate and error bars are SD. b M85151a has twice as many cell bound molecules at saturation as other anti-CEA antibodies. LS174T cells were labeled to saturation with Alexa-488 conjugated antibodies and the number of molecules bound per cell calculated as described in “Materials and methods”. Both the IgG and Fab versions of M85151a have approximately twice as many molecules bound at saturation as other antibodies of equivalent valency. All measurements done in triplicate and error bars are SD
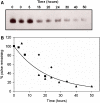
Fig. 6
Metabolic turnover of CEA. Cell surface proteins were pulsed with biotin using an NHS-SS-biotin reagent and chased at 37°C. At each time point, cells were lysed, biotinylated proteins pulled down with streptavidin resin, and the pulldown blotted for CEA as described in “Materials and methods”. Band intensities were normalized to the signal at time zero and fit to a negative exponential. a Western blot from a single experiment. b Pooled data from three separate experiments fit to a negative exponential. Each symbol in (b) represents a different experiment. CEA is degraded with a half-time of 15 h, similar to the internalization rate of the anti-CEA antibodies
Similar articles
- Targeting and therapy of carcinoembryonic antigen-expressing tumors in transgenic mice with an antibody-interleukin 2 fusion protein.
Xu X, Clarke P, Szalai G, Shively JE, Williams LE, Shyr Y, Shi E, Primus FJ. Xu X, et al. Cancer Res. 2000 Aug 15;60(16):4475-84. Cancer Res. 2000. PMID: 10969795 - Screening and kinetic analysis of recombinant anti-CEA antibody fragments.
Abraham R, Buxbaum S, Link J, Smith R, Venti C, Darsley M. Abraham R, et al. J Immunol Methods. 1995 Jun 14;183(1):119-25. doi: 10.1016/0022-1759(95)00039-d. J Immunol Methods. 1995. PMID: 7602129 - Efficient construction of a diabody using a refolding system: anti-carcinoembryonic antigen recombinant antibody fragment.
Asano R, Kudo T, Nishimura Y, Makabe K, Hayashi H, Suzuki M, Tsumoto K, Kumagai I. Asano R, et al. J Biochem. 2002 Dec;132(6):903-9. doi: 10.1093/oxfordjournals.jbchem.a003303. J Biochem. 2002. PMID: 12473192 - Pharmacokinetics and biodistribution of genetically-engineered antibodies.
Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Colcher D, et al. Q J Nucl Med. 1998 Dec;42(4):225-41. Q J Nucl Med. 1998. PMID: 9973838 Review. - Re-targeting of cytotoxic T lymphocytes and/or natural killer cells to CEA-expressing tumor cells with anti-CEA antibody activity.
Kuroki M, Hachimine K, Huang J, Shibaguchi H, Kinugasa T, Maekawa S, Kuroki M. Kuroki M, et al. Anticancer Res. 2005 Nov-Dec;25(6A):3725-32. Anticancer Res. 2005. PMID: 16302732 Review.
Cited by
- Local delivery of cell surface-targeted immunocytokines programs systemic antitumor immunity.
Santollani L, Maiorino L, Zhang YJ, Palmeri JR, Stinson JA, Duhamel LR, Qureshi K, Suggs JR, Porth OT, Pinney W 3rd, Msari RA, Walsh AA, Wittrup KD, Irvine DJ. Santollani L, et al. Nat Immunol. 2024 Oct;25(10):1820-1829. doi: 10.1038/s41590-024-01925-7. Epub 2024 Aug 7. Nat Immunol. 2024. PMID: 39112631 Free PMC article. - Universal CAR 2.0 to overcome current limitations in CAR therapy.
Schlegel LS, Werbrouck C, Boettcher M, Schlegel P. Schlegel LS, et al. Front Immunol. 2024 Jun 19;15:1383894. doi: 10.3389/fimmu.2024.1383894. eCollection 2024. Front Immunol. 2024. PMID: 38962014 Free PMC article. Review. - Local delivery of cell surface-targeted immunocytokines programs systemic anti-tumor immunity.
Santollani L, Zhang YJ, Maiorino L, Palmeri JR, Stinson JA, Duhamel LR, Qureshi K, Suggs JR, Porth OT, Pinney W 3rd, Msari RA, Wittrup KD, Irvine DJ. Santollani L, et al. bioRxiv [Preprint]. 2024 Jan 3:2024.01.03.573641. doi: 10.1101/2024.01.03.573641. bioRxiv. 2024. PMID: 38260254 Free PMC article. Updated. Preprint. - CT109-SN-38, a Novel Antibody-drug Conjugate with Dual Specificity for CEACAM5 and 6, Elicits Potent Killing of Pancreatic Cancer Cells.
Cardenas KCA, Enos CW, Spear MR, Austin DE, Almofeez R, Kortchak S, Pincus L, Guo HB, Dolezal S, Pierce JM, Furth E, Gineste C, Kwon Y, Gelber C. Cardenas KCA, et al. Curr Cancer Drug Targets. 2024;24(7):720-732. doi: 10.2174/0115680096260614231115192343. Curr Cancer Drug Targets. 2024. PMID: 38178674 - Improving Intracellular Delivery of an Antibody-Drug Conjugate Targeting Carcinoembryonic Antigen Increases Efficacy at Clinically Relevant Doses In Vivo.
Nessler I, Rubahamya B, Kopp A, Hofsess S, Cardillo TM, Sathyanarayan N, Donnell J, Govindan SV, Thurber GM. Nessler I, et al. Mol Cancer Ther. 2024 Mar 4;23(3):343-353. doi: 10.1158/1535-7163.MCT-23-0437. Mol Cancer Ther. 2024. PMID: 37913500 Free PMC article.
References
- Adams GP, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 2001;61:4750–4755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA101830-04/CA/NCI NIH HHS/United States
- T32 GM008334/GM/NIGMS NIH HHS/United States
- R01 CA096504-07/CA/NCI NIH HHS/United States
- CA101830/CA/NCI NIH HHS/United States
- R01 CA101830/CA/NCI NIH HHS/United States
- R01 CA096504/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources