The ubiquitin E3 ligase MARCH7 is differentially regulated by the deubiquitylating enzymes USP7 and USP9X - PubMed (original) (raw)
The ubiquitin E3 ligase MARCH7 is differentially regulated by the deubiquitylating enzymes USP7 and USP9X
James A Nathan et al. Traffic. 2008 Jul.
Abstract
Protein modification by one or more ubiquitin chains serves a critical signalling function across a wide range of cellular processes. Specificity within this system is conferred by ubiquitin E3 ligases, which target the substrates. Their activity is balanced by deubiquitylating enzymes (DUBs), which remove ubiquitin from both substrates and ligases. The RING-CH ligases were initially identified as viral immunoevasins involved in the downregulation of immunoreceptors. Their cellular orthologues, the Membrane-Associated RING-CH (MARCH) family represent a subgroup of the classical RING genes. Unlike their viral counterparts, the cellular RING-CH proteins appear highly regulated, and one of these in particular, MARCH7, was of interest because of a potential role in neuronal development and lymphocyte proliferation. Difficulties in detection and expression of this orphan ligase lead us to search for cellular cofactors involved in MARCH7 stability. In this study, we show that MARCH7 readily undergoes autoubiquitylation and associates with two deubiquitylating enzymes - ubiquitin-specific protease (USP)9X in the cytosol and USP7 in the nucleus. Exogenous expression and short interfering RNA depletion experiments demonstrate that MARCH7 can be stabilized by both USP9X and USP7, which deubiquitylate MARCH7 in the cytosol and nucleus, respectively. We therefore demonstrate compartment-specific regulation of this E3 ligase through recruitment of site-specific DUBs.
Figures
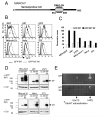
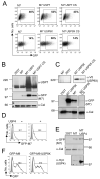
Figure 1. MARCH7 is preferentially expressed in cells of neuronal origin and is stabilised by functional mutations within the RING-CH domain
A) Schematic of MARCH7, showing the RING-CH and serine/proline rich N-terminus (hatched area). B-D) The indicated cell lines were transduced with lentivirus encoding GFP-MARCH7, GFP-MARCH7 WI or a control virus encoding GFP alone and after 3 days analysed by flow cytometry (B) or western blotting (D). The multiplicity of infection (MOI) was estimated for each cell line by titration of the control GFP virus, and kept constant for all cell lines. The mean fluorescent intensity (MFI) for GFP-MARCH7 (open bars) and GFP-MARCH7 WI (closed bars) are shown in (C). The transduced cell lines were lysed in RIPA buffer and immunoblotted with an anti-GFP antibody for MARCH7 (D). Twice as much lysate was loaded for the non-neuronal cell lines (Nthy-ori-3.1, HeLa and 3T3) compared to the neuronal cell lines (HEK-293T, U87 and U373) to allow detection of GFP-MARCH7 protein. E) MARCH7 recruits the E2 conjugating enzymes HIP2 and Ubc13. Full length human MARCH7 and MARCH7 WI were expressed in a yeast two hybrid assay against a library of human E2 conjugating enzymes. The yeast colonies were selected on histidine treated plates. Wildtype MARCH7 associated with HIP2 and Ubc13 (top panel) whilst the MARCH7 WI mutant is unable to bind the E2 conjugating enzymes Ubc13 and HIP2 (bottom panel). UbcH7 autoactivates on histidine treated plates. M7 MARCH7, M7 WI MARCH7 WI, Cal calreticulin.
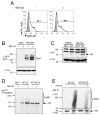
Figure 2. MARCH7 is ubiquitylated i_n vivo_
A-C) MARCH7 is stabilised with MG132 treatment. HEK-293T cells were transfected with MARCH7 or GFP-MARCH7 and incubated with 7.5μM MG132 or DMSO for 18 hours. GFP-MARCH7 expression was measured by flow cytometry and western blotting (A, B). Untagged MARCH7 expression was measured by immunoblotting using the chicken MARCH7 antisera (C). Calreticulin (Cal) was immunoblotted as a loading control. D, E) MARCH7 ubiquitylation. Radiolabeled MARCH7 detects higher molecular weight species consistent with ubiquitylation (D). HEK-293T cells were transfected with MARCH7-ZZ or MARCH7 WI-ZZ, radiolabelled with [35S] methionine and [35S] cysteine for 15 minutes and chased for 90 minutes. Cells were pretreated with 20μM MG132 or DMSO control from the starve until the end of the chase (150 minutes), lysed in RIPA buffer, immunoprecipitated on IgG sepharose beads and after SDS-PAGE separation, analysed by phosphoimager. E) HEK-293T cells co-transfected with or without HA tagged ubiquitin (HA-Ub) and either MARCH7-ZZ or MARCH7 WI-ZZ were lysed in RIPA buffer and MARCH7 immunoprecipitated by IgG pull down. Samples were separated by SDS-PAGE and immunoblotted for MARCH7 (c-myc antibody) and ubiquitin (HA antibody).
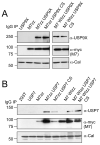
Figure 3. MARCH7 associates with the DUBs USP9X and USP7
A, B) MARCH7 co-immunoprecipitates with USP7 and USP9X. HEK-293T cells were co-transfected with MARCH7-ZZ/MARCH7 WI-ZZ and (A) USP9X/USP9X CS or (B) USP7/USP7 CS, lysed in 1% Triton X-100 and MARCH7 immunoprecipitated on IgG sepharose beads. 1×106 cells equivalents were loaded per lane, separated by SDS-PAGE and immunoblotted for USP9X, USP7 and MARCH7 (c-myc antibody). 1×105 cells/lane of lysate were loaded and immunoblotted for calreticulin (Cal) as a loading control.
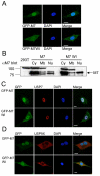
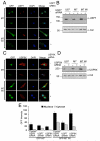
Figure 4. MARCH7 localises to the cytosol, nucleus and plasma membrane
A) MARCH7 and MARCH7 WI subcellular localisation. The U87 glioblastoma cell line was transduced with lentivirus encoding GFP-MARCH7 or GFP-MARCH7 WI (green). Cells were plated on coverslips and fixed with 4% paraformaldehyde after 3 days. DAPI was used as a nuclear stain (blue). B) MARCH7 is present in cytosolic, nuclear and membrane cellular fractions. HEK-293T cells were transfected with MARCH7 or MARCH7 WI and specific cellular fractions isolated after 48 hours, acetone precipitated, separated by SDS-PAGE and visualised with the chicken MARCH7 antisera. Five times more cell lysate was loaded in the MARCH7 transfected cells compared with the MARCH7 WI cells to allow comparison. C, D) MARCH7 co-localises with USP7 and USP9X. U87 cells were transduced with GFP-MARCH7 or GFP-MARCH7 WI lentivirus (green) and fixed on coverslips after 3 days. The coverslips were probed for USP7 (C) or USP9X (D) and detected using an anti-rabbit Alexafluor 568 antibody (red). DAPI (blue) was used as a nuclear counter stain. Bar=10μm Cy cytosolic fraction, Mb membrane fraction, Nu nuclear fraction
Figure 5. MARCH7 is stabilised by USP7 and USP9X
A-C) HEK-293T cells were co-transfected with GFP-MARCH7 and USP7 or USP7 CS (A, B) or GFP-MARCH7 and USP9X or USP9X CS (A, C). After 48 hours cells were harvested and analysed by flow cytometry (A) or western blotting (B, C). The percent GFP positive cells in the gated population out of the total number of live cells is indicated. Cell lysates were prepared using RIPA buffer and 1x105 cell equivalents loaded (B, C). Lysates were probed for MARCH7 (anti-GFP mAb.) (B and C, middle panels), USP7 (B, top panel) and USP9X (anti-V5 mAb.) (C, top panel). Calreticulin was blotted as control (B and C, bottom panels). D, E) USP4 has no effect on MARCH7 levels. HEK-293T cells were transfected with GFP-MARCH7 in the presence of myc tagged USP4 and analysed by flow cytometry (D). The shaded histogram shows the non-transfected population of cells. RIPA extracts were probed for MARCH7 (anti-GFP), USP4 (myc antibody - 9E10) and calreticulin (E). F) USP9X does not stabilise MARCH9. HEK-293T cells were co-transfected with GFP-MARCH9 and USP9X, and after 48 hours analysed by flow cytometry. The shaded histogram refers to the non-transfected control cells. Percentages refer to the gated GFP positive population.
Figure 6. SiRNA mediated depletion of USP9X and USP7 affects the stability of MARCH7
A, B) HEK-293T cells were transfected with siRNA specific for USP7, USP9X or USP8 (as a control). After 24 hours cells were transfected with GFP-MARCH7. Analysis by (A) flow cytometry or (B) immunoblotting was performed after a further 48 hours. The percentages refer to the geometric mean of GFP positive cells from the total live cell population. Cell lysates were prepared in RIPA buffer and blotted for endogenous USP7, USP9X and MARCH7 (GFP antibody). Calreticulin was used as a loading control.
Figure 7. MARCH7 is deubiquitylated by USP7 and USP9X
HEK-293T cells were co-transfected with ZZ-myc tagged MARCH7 or MARCH7 WI, HA tagged ubiquitin and either USP7 or USP9X. After 48 hours cells were lysed in RIPA buffer and MARCH7 immunoprecipitated on IgG sepharose beads. Membranes were probed for ubiquitin (HA antibody) and MARCH7 (myc antibody).
Figure 8. SiRNA mediated depletion of USP9X AND USP7 alters the subcellular localisation of MARCH7
A-D) U87 cells were transduced with GFP-MARCH7 or GFP-MARCH7 WI lentivirus and after 24 hours transfected with siRNA specific for USP7 or USP9X. Cells were analysed by confocal microscopy (A, C) and immunoblotting (B, D) after a further 72 hours. For microscopy, the U87 cells were plated onto coverslips, fixed and stained for USP7 (A) or USP9X (C) as above. RIPA extracted cell lysates were probed for endogenous USP7 (B) or USP9X (D). Calreticulin was used as a loading control. (E) Quantitative analysis of GFP-MARCH7 expression following siRNA mediated depletion of USP7 and USP9X demonstrates compartment-specific regulation of MARCH7. U87 cells were transduced with GFP-MARCH7 or GFP-MARCH7 WI and transfected with siRNA specific for USP8, USP7 and USP9X as described previously. 10 image fields (containing between 1-6 cells) for each siRNA mediated depletion were analysed for GFP-MARCH7 intensity levels in the nucleus and cytosol using Volocity software. The results are presented as the mean GFP intensity (arbitrary units), with the error bars indicating the standard error of the mean. Bar=10μm.
Similar articles
- Expression of USP7 and MARCH7 Is Correlated with Poor Prognosis in Epithelial Ovarian Cancer.
Zhang L, Wang H, Tian L, Li H. Zhang L, et al. Tohoku J Exp Med. 2016 Jul;239(3):165-75. doi: 10.1620/tjem.239.165. Tohoku J Exp Med. 2016. PMID: 27302477 - Deubiquitinase ubiquitin-specific protease 9X regulates the stability and function of E3 ubiquitin ligase ring finger protein 115 in breast cancer cells.
Lu Q, Lu D, Shao ZM, Li DQ. Lu Q, et al. Cancer Sci. 2019 Apr;110(4):1268-1278. doi: 10.1111/cas.13953. Epub 2019 Feb 21. Cancer Sci. 2019. PMID: 30689267 Free PMC article. - A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7.
Canning M, Boutell C, Parkinson J, Everett RD. Canning M, et al. J Biol Chem. 2004 Sep 10;279(37):38160-8. doi: 10.1074/jbc.M402885200. Epub 2004 Jul 6. J Biol Chem. 2004. PMID: 15247261 - A portrayal of E3 ubiquitin ligases and deubiquitylases in cancer.
Satija YK, Bhardwaj A, Das S. Satija YK, et al. Int J Cancer. 2013 Dec 15;133(12):2759-68. doi: 10.1002/ijc.28129. Epub 2013 Mar 25. Int J Cancer. 2013. PMID: 23436247 Review. - Ubiquitin becomes ubiquitous in cancer: emerging roles of ubiquitin ligases and deubiquitinases in tumorigenesis and as therapeutic targets.
Shi D, Grossman SR. Shi D, et al. Cancer Biol Ther. 2010 Oct 15;10(8):737-47. doi: 10.4161/cbt.10.8.13417. Epub 2010 Oct 15. Cancer Biol Ther. 2010. PMID: 20930542 Free PMC article. Review.
Cited by
- Ubiquitin E3 ligase MARCH7 promotes ovarian tumor growth.
Hu J, Meng Y, Yu T, Hu L, Mao M. Hu J, et al. Oncotarget. 2015 May 20;6(14):12174-87. doi: 10.18632/oncotarget.3650. Oncotarget. 2015. PMID: 25895127 Free PMC article. - Ubiquitin-independent degradation of antiapoptotic MCL-1.
Stewart DP, Koss B, Bathina M, Perciavalle RM, Bisanz K, Opferman JT. Stewart DP, et al. Mol Cell Biol. 2010 Jun;30(12):3099-110. doi: 10.1128/MCB.01266-09. Epub 2010 Apr 12. Mol Cell Biol. 2010. PMID: 20385764 Free PMC article. - Regulation of the Mdm2-p53 pathway by the ubiquitin E3 ligase MARCH7.
Zhao K, Yang Y, Zhang G, Wang C, Wang D, Wu M, Mei Y. Zhao K, et al. EMBO Rep. 2018 Feb;19(2):305-319. doi: 10.15252/embr.201744465. Epub 2018 Jan 2. EMBO Rep. 2018. PMID: 29295817 Free PMC article. - Membrane-associated RING-CH 10 (MARCH10 protein) is a microtubule-associated E3 ubiquitin ligase of the spermatid flagella.
Iyengar PV, Hirota T, Hirose S, Nakamura N. Iyengar PV, et al. J Biol Chem. 2011 Nov 11;286(45):39082-90. doi: 10.1074/jbc.M111.256875. Epub 2011 Sep 21. J Biol Chem. 2011. PMID: 21937444 Free PMC article. - Viral and cellular MARCH ubiquitin ligases and cancer.
Wang X, Herr RA, Hansen T. Wang X, et al. Semin Cancer Biol. 2008 Dec;18(6):441-50. doi: 10.1016/j.semcancer.2008.09.002. Epub 2008 Oct 2. Semin Cancer Biol. 2008. PMID: 18948196 Free PMC article. Review.
References
- Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. - PubMed
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. - PubMed
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33. - PubMed
- Lehner PJ, Hoer S, Dodd R, Duncan LM. Downregulation of cell surface receptors by the K3 family of viral and cellular ubiquitin E3 ligases. Immunol Rev. 2005;207:112–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G9800943/MRC_/Medical Research Council/United Kingdom
- G0600823/MRC_/Medical Research Council/United Kingdom
- 084957/WT_/Wellcome Trust/United Kingdom
- 077028/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G0600823(78905)/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous