Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system - PubMed (original) (raw)
Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system
Harjeet Singh et al. Cancer Res. 2008.
Abstract
Genetic modification of clinical-grade T cells is undertaken to augment function, including redirecting specificity for desired antigen. We and others have introduced a chimeric antigen receptor (CAR) to enable T cells to recognize lineage-specific tumor antigen, such as CD19, and early-phase human trials are currently assessing safety and feasibility. However, a significant barrier to next-generation clinical studies is developing a suitable CAR expression vector capable of genetically modifying a broad population of T cells. Transduction of T cells is relatively efficient but it requires specialized manufacture of expensive clinical grade recombinant virus. Electrotransfer of naked DNA plasmid offers a cost-effective alternative approach, but the inefficiency of transgene integration mandates ex vivo selection under cytocidal concentrations of drug to enforce expression of selection genes to achieve clinically meaningful numbers of CAR(+) T cells. We report a new approach to efficiently generating T cells with redirected specificity, introducing DNA plasmids from the Sleeping Beauty transposon/transposase system to directly express a CD19-specific CAR in memory and effector T cells without drug selection. When coupled with numerical expansion on CD19(+) artificial antigen-presenting cells, this gene transfer method results in rapid outgrowth of CD4(+) and CD8(+) T cells expressing CAR to redirect specificity for CD19(+) tumor cells.
Figures
Figure 1
Schematic of the expression plasmids and experimental design. A, CoOpCD19RCD28/pT-MNDU3 (Transposon). MNDU3 promoter, the constitutive promoter from the U3 region of the MND retrovirus; CoOp CD19RCD28, codon-optimized CD19RCD28 CAR; IR, _SB_-inverted/direct repeats; bGh pAn, polyadenylation signal from bovine growth hormone; AmpR, ampicillin resistance gene. B, pCMV-SB11 (Transposase). SB11, _SB_-transposase SB11; CMV promoter, CMV enhancer/promoter; SV40pAN, polyadenylation signals from SV40. C, CoOpCD19CD28/Hy-pVitro4. EF1α promoter, composite promoter comprising the elongation factor-1α (EF1α) core promoter and the R segment and part of the U5 sequence (R-U5′) of the human T-cell leukemia virus type 1 LTR; pMB1 ori, a minimal E. coli origin of replication; SpAn, synthetic pause; CAGp, a composite promoter that combines the human CMV immediate-early enhancer and a modified chicken β-actin promoter and first intron; Hy, hygromycin B resistance gene (hygromycin phosphotransferase); bGh pAn, polyadenylation signal from bovine growth hormone; EM7, synthetic prokaryotic promoter. D, an expression cassette in a plasmid (blue) provides only transient expression unless incorporated into an integrating transposon vector that can be cleaved from the plasmid and integrated into a host genome by a source of transposase (red).
Figure 2
Specificity of mouse-derived CAR-specific mAb (clone 2D3). A, Jurkat cells were genetically modified and sorted to express CD19R. Jurkat parental (gray line) and CD19R+ (black line) cells were stained with (i) Alexa 488–conjugated clone 2D3 and (ii) F(ab′)2-fragment of goat-derived polyclonal antibody specific for human Fc; iii, binding of 2D3 (solid line) was blocked by polyclonal Fc-specific antisera (dashed line). B, cell surface staining of Alexa Fluor 488–conjugated clone 2D3 by confocal microscopy on (i) CD19R+ Jurkat cells and (ii) Jurkat parental cells. Cells were stained, fixed, and mounted as described in Materials and Methods.
Figure 3
Characterization of CAR expression on peripheral blood–derived T cells after electrotransfer of SB plasmid system. A, expression of CAR on CD8+ and CD4+ T cells after electrotransfer of SB transposon with or without SB11 transposase at 24 h, and 4 and 5 wk of coculture on g-irradiated K562-derived aAPC expressing tCD19, IL-15-Fc, MICA, and 4−1BBL. B, i, immunophenotype of memory cell markers (CD27, CD28, CD62L) on genetically modified T cells generated after 5 wk of coculture on aAPC. The gray-filled histograms reveal the percentage of T cells expressing CD27, CD28, and CD62L in the lymphocyte-gated population. Those expressing the memory cell markers were analyzed for coexpression of CAR (detected by mAb clone 2D3) and CD8 or CD4. ii, expression of CD45RO, CD45RA, and CD62L on T cells generated after coculture. CAR+ CD4 or CD8 cells were analyzed for the expression of CD45RA and CD45RO. The MFI of the unmanipulated T cells was 867/50 (CD45RA/CD45RO) compared with 28/38 for the SB-transfected T cells. CD45RO and CD62L double-positive cells were also analyzed for coexpression of CAR. iii, TCM, defined as double-positive for CD28 and CD95 (TEM, CD28negCD95pos), were analyzed for coexpression of CD62L and CAR. C, Western blot analysis of CAR expression detected by mAb specific for CD3-ζ. Whole-cell protein (20 μg) lysates from primary T cells genetically modified with CoOpCD19RCD28 (lane 1, ∼79 kDa chimeric protein) or no plasmid control (lane 2); CD19R+ Jurkat cells (lane 3, ∼66 kDa chimeric protein) or parental Jurkat (lane 4) were resolved by SDS-PAGE under reducing conditions. D, integration of CoOpCD19RCD28 by PCR. DNA was isolated from T cells after mock electroporation (no DNA, lanes 1 and 4), from T cells 28 d after electroporation with SB transposon in the absence of transposase (lanes 2 and 5), and from T cells 28 d after electroporation with transposon in the presence of SB11 transposase (lanes 3 and 6). PCR was accomplished using transposon-specific primers (lanes 1–3) or GAPDH-specific primers (lanes 4−6). The data showing SB system in peripheral blood/cord blood are from a representative experiment.
Figure 4
Sustained proliferation of genetically modified primary peripheral blood–derived T cells with analysis of CAR expression and TCR repertoire. A, kinetics of propagation of T cells in culture with aAPC. The average T-cell numerical expansion was 22-fold (range 20−31) every 7 d for up to 10 wk of continuous coculture with aAPC. B, percent expression (unbroken lines, left axis) and density as measured by MFI (dotted line, right axis) of CAR on total, CD4+, CD8+ T cells as measured by flow cytometry over culture time on aAPC. C, TCR Vβ analysis by flow cytometry 4 wk after electrotransfer of SB plasmids (filled columns) or CoOpCD19CD28/Hy-pVitro4 plasmid (open columns). Data are representative of two different experiments.
Figure 5
Redirected specificity of peripheral blood–derived T cells genetically modified with SB system. A, killing of CD19+ target cells (HLA Ineg Daudi; HLA class I/IIneg K562 cells transfected to express truncated CD19) in a standard 4-h chromium release assay. Background lysis of CD19neg (parental K562) cells is shown. Points, mean specific lysis of triplicate wells at effector to target (E:T) cell ratios; bars, SD. B, CAR and intracellular IL-2 expression after incubating with panel of stimulator cells by multiparameter flow cytometry gating on CD3+ lymphocytes. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were added as a positive control.
Figure 6
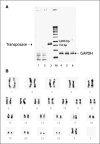
Safety issues regarding SB transposase and chromosomal aberrations. A, lack of integration of SB11 transposase by genomic PCR from genetically modified and propagated peripheral blood–derived T cells. DNA was isolated from T cells after mock electroporation (no DNA, lanes 1 and 4), from T cells 28 d after electroporation with the two-plasmid SB system (lanes 2 and 5), or from T cells 1 d after electroporation with the two-plasmid SB system (lanes 3 and 6). PCR was accomplished using transposase-specific primers (lanes 1−3) or GAPDH-specific primers (lanes 4−6). B, idiogram of a G-banded karyotype of the _SB_-transfected peripheral blood–derived T cells showing no apparent numerical or structural chromosome alterations.
Similar articles
- Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells.
Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, Maiti S, Manuri P, Senyukov V, Jena B, Kebriaei P, Champlin RE, Huls H, Cooper LJ. Singh H, et al. PLoS One. 2013 May 31;8(5):e64138. doi: 10.1371/journal.pone.0064138. Print 2013. PLoS One. 2013. PMID: 23741305 Free PMC article. - Sleeping beauty system to redirect T-cell specificity for human applications.
Maiti SN, Huls H, Singh H, Dawson M, Figliola M, Olivares S, Rao P, Zhao YJ, Multani A, Yang G, Zhang L, Crossland D, Ang S, Torikai H, Rabinovich B, Lee DA, Kebriaei P, Hackett P, Champlin RE, Cooper LJ. Maiti SN, et al. J Immunother. 2013 Feb;36(2):112-23. doi: 10.1097/CJI.0b013e3182811ce9. J Immunother. 2013. PMID: 23377665 Free PMC article. - Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood.
Huls MH, Figliola MJ, Dawson MJ, Olivares S, Kebriaei P, Shpall EJ, Champlin RE, Singh H, Cooper LJ. Huls MH, et al. J Vis Exp. 2013 Feb 1;(72):e50070. doi: 10.3791/50070. J Vis Exp. 2013. PMID: 23407473 Free PMC article. - A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19.
Singh H, Huls H, Kebriaei P, Cooper LJ. Singh H, et al. Immunol Rev. 2014 Jan;257(1):181-90. doi: 10.1111/imr.12137. Immunol Rev. 2014. PMID: 24329797 Free PMC article. Review. - Manufacture of T cells using the Sleeping Beauty system to enforce expression of a CD19-specific chimeric antigen receptor.
Singh H, Moyes JS, Huls MH, Cooper LJ. Singh H, et al. Cancer Gene Ther. 2015 Mar;22(2):95-100. doi: 10.1038/cgt.2014.69. Epub 2015 Jan 16. Cancer Gene Ther. 2015. PMID: 25591810 Review.
Cited by
- Genetically modified and unmodified cellular approaches to enhance graft versus leukemia effect, without increasing graft versus host disease: the use of allogeneic cytokine-induced killer cells.
Rambaldi B, Rizzuto G, Rambaldi A, Introna M. Rambaldi B, et al. Front Immunol. 2024 Oct 24;15:1459175. doi: 10.3389/fimmu.2024.1459175. eCollection 2024. Front Immunol. 2024. PMID: 39512351 Free PMC article. Review. - Cancer stem cells: advances in knowledge and implications for cancer therapy.
Chu X, Tian W, Ning J, Xiao G, Zhou Y, Wang Z, Zhai Z, Tanzhu G, Yang J, Zhou R. Chu X, et al. Signal Transduct Target Ther. 2024 Jul 5;9(1):170. doi: 10.1038/s41392-024-01851-y. Signal Transduct Target Ther. 2024. PMID: 38965243 Free PMC article. Review. - Acute lymphoblastic leukaemia.
Pagliaro L, Chen SJ, Herranz D, Mecucci C, Harrison CJ, Mullighan CG, Zhang M, Chen Z, Boissel N, Winter SS, Roti G. Pagliaro L, et al. Nat Rev Dis Primers. 2024 Jun 13;10(1):41. doi: 10.1038/s41572-024-00525-x. Nat Rev Dis Primers. 2024. PMID: 38871740 Review. - Evolution of the clinical-stage hyperactive TcBuster transposase as a platform for robust non-viral production of adoptive cellular therapies.
Skeate JG, Pomeroy EJ, Slipek NJ, Jones BJ, Wick BJ, Chang JW, Lahr WS, Stelljes EM, Patrinostro X, Barnes B, Zarecki T, Krueger JB, Bridge JE, Robbins GM, McCormick MD, Leerar JR, Wenzel KT, Hornberger KM, Walker K, Smedley D, Largaespada DA, Otto N, Webber BR, Moriarity BS. Skeate JG, et al. Mol Ther. 2024 Jun 5;32(6):1817-1834. doi: 10.1016/j.ymthe.2024.04.024. Epub 2024 Apr 16. Mol Ther. 2024. PMID: 38627969 - Mage transposon: a novel gene delivery system for mammalian cells.
Tian J, Tong D, Li Z, Wang E, Yu Y, Lv H, Hu Z, Sun F, Wang G, He M, Xia T. Tian J, et al. Nucleic Acids Res. 2024 Mar 21;52(5):2724-2739. doi: 10.1093/nar/gkae048. Nucleic Acids Res. 2024. PMID: 38300794 Free PMC article.
References
- Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–44. - PubMed
- Jensen MC, Popplewell L, DiGiusto DL, et al. A first-n-human clinical trial of adoptive therapy using CD19-specific chimeric antigen receptor re-directed T cells for recurrent/refractory follicular lymphoma. Mol Ther. 2007;15:S142.
- Kowolik CM, Topp MS, Gonzalez S, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA141303/CA/NCI NIH HHS/United States
- R21 CA116127-02/CA/NCI NIH HHS/United States
- P30 CA016672-24/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- CA116127/CA/NCI NIH HHS/United States
- P01 HD032652-130006/HD/NICHD NIH HHS/United States
- R01 CA120956-02/CA/NCI NIH HHS/United States
- R21 CA129390-01/CA/NCI NIH HHS/United States
- R21 CA116127-01A1/CA/NCI NIH HHS/United States
- R21 CA116127/CA/NCI NIH HHS/United States
- R01 CA120956-01A1/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA124782/CA/NCI NIH HHS/United States
- CA120956/CA/NCI NIH HHS/United States
- R01 CA124782-01A1/CA/NCI NIH HHS/United States
- R21 CA129390-02/CA/NCI NIH HHS/United States
- R21 CA129390/CA/NCI NIH HHS/United States
- P01 HD032652-08S10006/HD/NICHD NIH HHS/United States
- R33 CA116127/CA/NCI NIH HHS/United States
- R01 CA120956/CA/NCI NIH HHS/United States
- R01 CA124782-02/CA/NCI NIH HHS/United States
- P01 HD032652/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials