UBE1L represses PML/RAR{alpha} by targeting the PML domain for ISG15ylation - PubMed (original) (raw)
UBE1L represses PML/RAR{alpha} by targeting the PML domain for ISG15ylation
Sumit J Shah et al. Mol Cancer Ther. 2008 Apr.
Abstract
Acute promyelocytic leukemia (APL) is characterized by expression of promyelocytic leukemia (PML)/retinoic acid (RA) receptor alpha (RARalpha) protein and all-trans-RA-mediated clinical remissions. RA treatment can confer PML/RARalpha degradation, overcoming dominant-negative effects of this oncogenic protein. The present study uncovered independent retinoid degradation mechanisms, targeting different domains of PML/RARalpha. RA treatment is known to repress PML/RARalpha and augment ubiquitin-activating enzyme-E1-like (UBE1L) protein expression in NB4-S1 APL cells. We previously reported RA-induced UBE1L and the IFN-stimulated gene, 15-kDa protein ISG15ylation in APL cells. Whether the ubiquitin-like protein ISG15 directly conjugates with PML/RARalpha was not explored previously and is examined in this study. Transient transfection experiments with different PML/RARalpha domains revealed that RA treatment preferentially down-regulated the RARalpha domain, whereas UBE1L targeted the PML domain for repression. As expected, ubiquitin-specific protease 18 (UBP43/USP18), the ISG15 deconjugase, opposed UBE1L but not RA-dependent PML/RARalpha degradation. In contrast, the proteasomal inhibitor, N-acetyl-leucinyl-leucinyl-norleucinal, inhibited both UBE1L- and RA-mediated PML/RARalpha degradation. Notably, UBE1L induced ISG15ylation of the PML domain of PML/RARalpha, causing its repression. These findings confirmed that RA triggers PML/RARalpha degradation through different domains and distinct mechanisms. Taken together, these findings advance prior work by establishing two pathways converge on the same oncogenic protein to cause its degradation and thereby promote antineoplastic effects. The molecular pharmacologic implications of these findings are discussed.
Figures
Figure 1
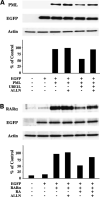
Immunoblot analyses of whole-cell lysates of NB4-S1 APL cells following RA treatment. A, NB4-S1 (2 × 106) cells per experimental group were treated with RA (1 μmol/L) for 0 to 48 h and immunoblot analyses were done to assess effects of RA treatment on expression of PML/RARα and UBE1L. B, scanning densitometric analyses were done for the immunoblots displayed in A. Expression of PML/RARα and UBE1L was each normalized for actin expression, which served as a loading control. This study confirmed induction of UBE1L concurrent with PML/RARα repression following RA treatment of NB4-S1 APL cells. That UBE1L targeted the PML domain of PML/RARα was shown. C, schematic representation of five different PML/RARα expression constructs designated 1 to 5. Gray box, PML domain; black box, RARα domain. Construct 1 depicted PML/RARα (S), which differs from the PML/RARα (L) depicted in construct 2 in that it does not contain the aspartate residue at position 522 (black diamond), the known caspase-3 cleavage site (16). Expression vectors were also engineered to independently contain a portion of the PML domain (construct 3), a portion of the RARα domain (construct 4), or the entire RARα domain (construct 5) of PML/RARα. D, immunoblot analyses for UBE1L and UBE1L-mediated repression of protein products of constructs 1 to 5. Each construct was cotransfected with the UBE1L expression plasmid (+) or with an insertless control vector (−) in BEAS-2B cells followed by immunoblot analyses. Cotransfection of the EGFP expression vector served as a transfection control. Actin expression confirmed similar amounts of protein lysates were loaded. Expression levels of transfected UBE1L are displayed as are the indicated quantified signals. This experiment revealed that UBE1L mediates PML/RARα repression by targeting the PML domain of PML/RARα. PML/RARα (L) construct 2, PML domain construct 3, and RARα domain construct 5 were used for subsequent experiments.
Figure 2
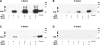
RA and UBE1L affect different PML/RARα domains. BEAS-2B cells were independently transfected with (A) PML/RARα, (B) PML, or (C) RARα expression constructs as described in Materials and Methods. Indicated groups were cotransfected with or without UBE1L and then treated for 24 additional hours with or without RA (1 μmol/L). Immunoblot analyses identified different domains of PML/RARα as being individually targeted by RA and UBE1L for repression of PML/RARα. Bottom, quantification of each of these signals.
Figure 3
Independent effects of UBP43 on RA- and UBE1L-mediated repression of PML/RARα and its domains. BEAS-2B cells were independently transfected with (A) PML/RARα, (B) PML, or (C) RARα domain containing expression vectors. Indicated groups were either cotransfected with UBE1L or treated for 24 h with vehicle (DMSO) or RA (1 μmol/L). Cells were also cotransfected with or without a UBP43 expression vector as indicated. Immunoblot analyses revealed that UBP43 preferentially opposes UBE1L- but not RA-mediated repression of the indicated PML/RARα species. Bottom, quantification of each of these signals.
Figure 4
Effects of proteasome inhibition by ALLN on RA- and UBE1L-mediated repression of PML/RARα domains, respectively. BEAS-2B cells were independently transfected with (A) PML or (B) RARα domain containing expression constructs. Indicated groups were also cotransfected with or without UBE1L. Following transfection, cells in the ALLN treatment groups were incubated with ALLN (100 μmol/L) for 90 additional minutes. RA (1 μmol/L) was then added to the respective RA treatment groups and incubated for 6 additional h. Remaining groups were incubated with the vehicle (DMSO) during this same treatment period. Immunoblot analyses revealed that ALLN inhibited RA-dependent repression of the RARα domain and UBE1L-dependent repression of the PML domain of PML/RARα. Bottom, quantification of each of these signals.
Figure 5
Effects of UBE1L on ISG15ylation of PML/RARα. BEAS-2B cells were individually transfected with PML/RARα containing expression constructs. Indicated groups were cotransfected with or without UBE1L. Following transfection, respective groups were treated with either ALLN (100 μmol/L) or vehicle (DMSO) for 4 (A and B) or 6 (C and D) additional hours. Immunoprecipitation followed by immunoblot analyses were done as described in Materials and Methods. The filters were probed with either a monoclonal anti-HA antibody to detect PML/RARα (A and C) or a monoclonal anti-ISG15 antibody to detect ISG15ylated forms of PML/RARα (B and D). Open arrows, positions of unconjugated PML/RARα proteins; closed arrows, positions of post-translational modification products of PML/RARα; Control, whole-cell lysates of COS-1 cells transfected with PML/RARα serve as a signal for unconjugated PML/RARα protein. These experiments established that UBE1L confers ISG15 conjugation to PML/RARα with subsequent repression of this protein.
Figure 6
Effects of UBE1L on ISG15ylation of the PML domain of PML/RARα. BEAS-2B cells were individually transfected with the PML domain fragment of PML/RARα containing expression construct. Indicated groups were cotransfected with or without UBE1L. Following transfection, respective groups were treated with either ALLN (100 μmol/L) or vehicle (DMSO) for 4 (A and B) or 6 (C and D) additional hours. Immunoprecipitation followed by immunoblot analyses were done as described in Materials and Methods. The filters were probed with either a monoclonal anti-HA antibody to detect PML (A and C) or a monoclonal anti-ISG15 antibody to detect ISG15ylated forms of PML (B and D). Open arrows, positions of unconjugated PML protein; closed arrows, positions of post-translational modification products; Control, whole-cell lysates of COS-1 cells transfected with PML serve as a signal for the unconjugated form of PML. These experiments established that UBE1L confers ISG15 conjugation to the PML domain of PML/RARα with subsequent repression of this protein.
Similar articles
- UBE1L is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia.
Kitareewan S, Pitha-Rowe I, Sekula D, Lowrey CH, Nemeth MJ, Golub TR, Freemantle SJ, Dmitrovsky E. Kitareewan S, et al. Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):3806-11. doi: 10.1073/pnas.052011299. Epub 2002 Mar 12. Proc Natl Acad Sci U S A. 2002. PMID: 11891284 Free PMC article. - Blockade of the ubiquitin protease UBP43 destabilizes transcription factor PML/RARα and inhibits the growth of acute promyelocytic leukemia.
Guo Y, Dolinko AV, Chinyengetere F, Stanton B, Bomberger JM, Demidenko E, Zhou DC, Gallagher R, Ma T, Galimberti F, Liu X, Sekula D, Freemantle S, Dmitrovsky E. Guo Y, et al. Cancer Res. 2010 Dec 1;70(23):9875-85. doi: 10.1158/0008-5472.CAN-10-1100. Epub 2010 Oct 8. Cancer Res. 2010. PMID: 20935222 Free PMC article. - Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia.
Pitha-Rowe I, Hassel BA, Dmitrovsky E. Pitha-Rowe I, et al. J Biol Chem. 2004 Apr 30;279(18):18178-87. doi: 10.1074/jbc.M309259200. Epub 2004 Feb 18. J Biol Chem. 2004. PMID: 14976209 - Molecular mechanisms of the antileukemia activities of retinoid and arsenic.
Nitto T, Sawaki K. Nitto T, et al. J Pharmacol Sci. 2014;126(3):179-85. doi: 10.1254/jphs.14r15cp. Epub 2014 Oct 15. J Pharmacol Sci. 2014. PMID: 25319615 Review. - Pathways of retinoic acid- or arsenic trioxide-induced PML/RARalpha catabolism, role of oncogene degradation in disease remission.
Zhu J, Lallemand-Breitenbach V, de Thé H. Zhu J, et al. Oncogene. 2001 Oct 29;20(49):7257-65. doi: 10.1038/sj.onc.1204852. Oncogene. 2001. PMID: 11704854 Review.
Cited by
- PMLRARα binds to Fas and suppresses Fas-mediated apoptosis through recruiting c-FLIP in vivo.
Tao RH, Berkova Z, Wise JF, Rezaeian AH, Daniluk U, Ao X, Hawke DH, Karp JE, Lin HK, Molldrem JJ, Samaniego F. Tao RH, et al. Blood. 2011 Sep 15;118(11):3107-18. doi: 10.1182/blood-2011-04-349670. Epub 2011 Jul 29. Blood. 2011. PMID: 21803845 Free PMC article. - Targeting promyelocytic leukemia protein: a means to regulating PML nuclear bodies.
Reineke EL, Kao HY. Reineke EL, et al. Int J Biol Sci. 2009 May 22;5(4):366-76. doi: 10.7150/ijbs.5.366. Int J Biol Sci. 2009. PMID: 19471587 Free PMC article. Review. - The Ubiquitin-Specific Peptidase USP18 Promotes Lipolysis, Fatty Acid Oxidation, and Lung Cancer Growth.
Liu X, Lu Y, Chen Z, Liu X, Hu W, Zheng L, Chen Y, Kurie JM, Shi M, Mustachio LM, Adresson T, Fox S, Roszik J, Kawakami M, Freemantle SJ, Dmitrovsky E. Liu X, et al. Mol Cancer Res. 2021 Apr;19(4):667-677. doi: 10.1158/1541-7786.MCR-20-0579. Epub 2020 Dec 30. Mol Cancer Res. 2021. PMID: 33380466 Free PMC article. - ISG15 in antiviral immunity and beyond.
Perng YC, Lenschow DJ. Perng YC, et al. Nat Rev Microbiol. 2018 Jul;16(7):423-439. doi: 10.1038/s41579-018-0020-5. Nat Rev Microbiol. 2018. PMID: 29769653 Free PMC article. Review. - Evidence for the ISG15-Specific Deubiquitinase USP18 as an Antineoplastic Target.
Mustachio LM, Lu Y, Kawakami M, Roszik J, Freemantle SJ, Liu X, Dmitrovsky E. Mustachio LM, et al. Cancer Res. 2018 Feb 1;78(3):587-592. doi: 10.1158/0008-5472.CAN-17-1752. Epub 2018 Jan 17. Cancer Res. 2018. PMID: 29343520 Free PMC article. Review.
References
- Frankel SR, Miller WH, Jr., Dmitrovsky E. Retinoic acid and its rearranged receptor in the etiology and treatment of acute promyelocytic leukemia. Oncology. 1992;6:74–8. - PubMed
- Xiao YH, Miller WH, Jr., Warrell RP, Dmitrovsky E, Zelenetz AD. Pulsed-field gel electrophoresis analysis of retinoic acid receptor-α and promyelocytic leukemia rearrangements. Detection of the t(15;17) translocation in the diagnosis of acute promyelocytic leukemia. Am J Pathol. 1993;143:1301–11. - PMC - PubMed
- Kakizuka A, Miller WH, Jr., Umesono K, et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell. 1991;66:663–74. - PubMed
- Warrell RP, Jr., Frankel SR, Miller WH, Jr., et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med. 1991;324:1385–93. - PubMed
- Yoshida H, Kitamura K, Tanaka K, et al. Accelerated degradation of PML-retinoic acid receptor α (PML-RARA) oncoprotein by all-trans-retinoic acid in acute promyelocytic leukemia: possible role of the proteasome pathway. Cancer Res. 1996;56:2945–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-CA087546/CA/NCI NIH HHS/United States
- R01 CA111422/CA/NCI NIH HHS/United States
- R01 CA087546/CA/NCI NIH HHS/United States
- T32-CA00959/CA/NCI NIH HHS/United States
- R01-CA062275/CA/NCI NIH HHS/United States
- R01 CA062275/CA/NCI NIH HHS/United States
- R01-CA111422/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous