Essential role of the chaperonin folding compartment in vivo - PubMed (original) (raw)
Essential role of the chaperonin folding compartment in vivo
Yun-Chi Tang et al. EMBO J. 2008.
Abstract
The GroEL/GroES chaperonin system of Escherichia coli forms a nano-cage allowing single protein molecules to fold in isolation. However, as the chaperonin can also mediate folding independently of substrate encapsulation, it remained unclear whether the folding cage is essential in vivo. To address this question, we replaced wild-type GroEL with mutants of GroEL having either a reduced cage volume or altered charge properties of the cage wall. A stepwise reduction in cage size resulted in a gradual loss of cell viability, although the mutants bound non-native protein efficiently. Strikingly, a mild reduction in cage size increased the yield and the apparent rate of green fluorescent protein folding, consistent with the view that an effect of steric confinement can accelerate folding. As shown in vitro, the observed acceleration of folding was dependent on protein encapsulation by GroES but independent of GroES cycling regulated by the GroEL ATPase. Altering the net-negative charge of the GroEL cage wall also strongly affected chaperonin function. Based on these findings, the GroEL/GroES compartment is essential for protein folding in vivo.
Figures
Figure 1
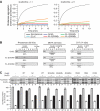
In vivo functionality of GroEL cavity-mutants. Constructs encoding the proteins indicated were transformed into E. coli MC4100 SC3 Kan R cells. Cells were grown in the presence of arabinose for expression of WT-GroEL/GroES. Serial dilutions corresponding to cell numbers indicated were plated on arabinose-containing plates for continued expression of WT-GroEL/GroES or IPTG-containing plates for expression of GroEL-mutants/GroES at 37°C as described in Materials and methods.
Figure 2
Substrate binding and _trans_-folding of GroEL cavity-mutants. (A) Prevention of rhodanese (Rho) aggregation in vitro was measured at an equimolar ratio of GroEL/rhodanese or at a twofold molar excess of GroEL (see Materials and methods). Aggregation after 10 min of rhodanese dilution from denaturant in the absence of chaperonin was set to 1. (B) Binary complexes of GroEL and GroEL-mutants with rhodanese, METK or SYT, produced by dilution of the denatured substrate proteins into GroEL-containing buffer, were analysed by size-exclusion chromatography. GroEL-bound substrates were quantified by immunoblotting with the loading control (L) set to 100%. Fractionation of GroEL and free native substrate proteins is indicated. (C) Capacity of GroEL cavity-mutants to support the soluble expression of the _trans_-folding substrate, yeast aconitase. Aconitase was overexpressed in E. coli BL21 cells with or without co-overexpression of WT-GroEL and GroEL-mutants with or without GroES at 37°C as indicated. Total (T), supernatant (S) and pellet (P) fractions of cells were analysed on SDS–PAGE and aconitase was quantified by densitometry. Standard deviations of at least three independent experiments are shown.
Figure 3
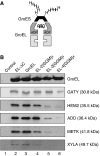
Encapsulation efficiency of endogenous substrates by GroEL cavity size-mutants. (A) Schematic representation of complexes of GroEL with His6-tagged _Mm_GroES arrested in the ADP state with substrate enclosed within the _cis_-cavity. (B) E. coli MC4100 cells overexpressing GroEL cavity size-mutants and His6-tagged _Mm_GroES were lysed, and chaperonin complexes containing endogenous substrates were isolated as described in Materials and methods. The substrate proteins indicated were detected by immunoblotting. GroEL was detected with the anti-serum against XYLA, which has strong GroEL cross-reactivity. To control for specificity, the same isolation procedure was performed with cells overexpressing WT-GroEL and non-His6-tagged _Mm_GroES.
Figure 4
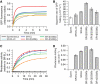
Enhanced folding of GFP upon mild reduction of GroEL cavity size. Solubility (A) and fluorescence (B) of WT-GFP upon co-overexpression with GroEL cavity size-mutants and GroES in E. coli MC4100 cells at 30°C. Cells were grown and analysed as in Figure 2C (see Materials and methods). Total (T), supernatant (S) and pellet (P) fractions from equal amounts of cells were analysed by SDS–PAGE and Coomassie staining. GFP fluorescence was measured in cell lysates containing equal amounts of total protein with the activity in the vector only control set to 1. Standard deviations of at least three independent experiments are shown. (C) and (D) Kinetics and yield of GFP refolding with GroEL cavity size-mutants and GroES in vitro. Refolding yields are plotted with the native GFP control set to 1. Refolding traces were fitted to a double exponential equation and apparent rates are plotted as the weighted average of the slow and fast rates based on their relative amplitudes. Standard deviations of at least three independent experiments are shown.
Figure 5
Motility and protease protection of substrate protein upon encapsulation in SR-EL cavity size-mutants. Steady-state fluorescence anisotropy of GFP (A) and DHFR–GFP fusion protein (B) upon addition of GroES and AMP-PNP to binary SR-EL-substrate complexes. Anisotropy values of native and spontaneously refolded proteins are shown as controls (see Materials and methods). Standard deviations of at least three independent experiments are shown. (C) and (D) Protease protection of DHFR–GFP upon addition of GroES and AMP-PNP to binary SR-EL-substrate complexes. Proteinase K (PK) protected DHFR–GFP was detected by immunoblotting with anti-GFP antibody and quantified by densitometry. Standard deviations of three independent experiments are shown.
Figure 6
Accelerated folding of GFP and rhodanese in cavity size-mutants is independent of ongoing ATP-hydrolysis. Kinetics and yield of WT-GFP refolding (A, B) and of rhodanese refolding (C, D) with SR-EL cavity size-mutants and GroES/ATP in vitro. Refolding yields are plotted with the native GFP and rhodanese control set to 1, respectively. Refolding traces for GFP were fitted as in Figure 4D and refolding traces for rhodanese were fitted to a single exponential equation. Note that there is essentially no spontaneous renaturation of rhodanese under the experimental conditions. Standard deviations of at least three independent experiments are shown.
Figure 7
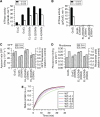
Accelerated folding by GroEL cavity size-mutants is independent of the rate of ATP hydrolysis. Steady-state ATPase activities of GroEL cavity size-mutants (A) and ATPase-deficient GroEL(D398A) cavity size-mutants (B) at 25°C. ATPase rates are indicated in ATP hydrolyzed per GroEL tetradecamer per min (see Materials and methods). Refolding yields and rates of WT-GFP (C) and rhodanese (D) with GroEL(D398A) cavity size-mutants and GroES/ATP. Refolding traces for GFP were fitted as in Figure 4D and refolding traces for rhodanese were fitted to a single exponential equation. The refolding yield obtained with WT-GroEL/GroES was set to 1. Standard deviations of at least three independent experiments are shown. (E) Simulation of rhodanese folding kinetics dependent on GroEL/GroES cycling rate at excess chaperonin over substrate. The binding rate of unfolded protein to GroEL was set to 2 × 107 M−1 s−1 (Rye et al, 1999) and binding of GroES to GroEL-substrate complexes was set to 1 × 106 M−1 s−1 (KC and SS, unpublished data, 2007). The rate of rhodanese refolding with WT-GroEL/GroES or SR-EL/GroES was set to 2.5 × 10−3 s−1 (Figure 7D). The normal ATPase-induced cycling rate was fixed at 0.07 s−1 with an approximate half-time of 10 s. The simulation was performed using chemical kinetics simulator (CKS) (
http://www.almaden.ibm.com/st/computational\_science/ck
). The ATPase-induced cycling rate was varied between 0.1- and 20-fold of the normal rate and refolding rates are plotted. The concentrations used for the simulation were GroEL 1 μM, GroES 2 μM and rhodanese 0.5 μM (see Supplementary Figure S5 for a kinetic model for the simulation).
Similar articles
- Active cage mechanism of chaperonin-assisted protein folding demonstrated at single-molecule level.
Gupta AJ, Haldar S, Miličić G, Hartl FU, Hayer-Hartl M. Gupta AJ, et al. J Mol Biol. 2014 Jul 29;426(15):2739-54. doi: 10.1016/j.jmb.2014.04.018. Epub 2014 May 6. J Mol Biol. 2014. PMID: 24816391 - Effective ATPase activity and moderate chaperonin-cochaperonin interaction are important for the functional single-ring chaperonin system.
Illingworth M, Salisbury J, Li W, Lin D, Chen L. Illingworth M, et al. Biochem Biophys Res Commun. 2015 Oct 9;466(1):15-20. doi: 10.1016/j.bbrc.2015.08.034. Epub 2015 Aug 11. Biochem Biophys Res Commun. 2015. PMID: 26271593 - Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein.
Tang YC, Chang HC, Roeben A, Wischnewski D, Wischnewski N, Kerner MJ, Hartl FU, Hayer-Hartl M. Tang YC, et al. Cell. 2006 Jun 2;125(5):903-14. doi: 10.1016/j.cell.2006.04.027. Cell. 2006. PMID: 16751100 - The GroEL-GroES Chaperonin Machine: A Nano-Cage for Protein Folding.
Hayer-Hartl M, Bracher A, Hartl FU. Hayer-Hartl M, et al. Trends Biochem Sci. 2016 Jan;41(1):62-76. doi: 10.1016/j.tibs.2015.07.009. Epub 2015 Sep 25. Trends Biochem Sci. 2016. PMID: 26422689 Review. - GroEL and the GroEL-GroES Complex.
Ishii N. Ishii N. Subcell Biochem. 2017;83:483-504. doi: 10.1007/978-3-319-46503-6_17. Subcell Biochem. 2017. PMID: 28271487 Review.
Cited by
- A Mutant Chaperonin That Is Functional at Lower Temperatures Enables Hyperthermophilic Archaea To Grow under Cold-Stress Conditions.
Gao L, Imanaka T, Fujiwara S. Gao L, et al. J Bacteriol. 2015 Aug;197(16):2642-52. doi: 10.1128/JB.00279-15. Epub 2015 May 26. J Bacteriol. 2015. PMID: 26013483 Free PMC article. - FKBP10 depletion enhances glucocerebrosidase proteostasis in Gaucher disease fibroblasts.
Ong DS, Wang YJ, Tan YL, Yates JR 3rd, Mu TW, Kelly JW. Ong DS, et al. Chem Biol. 2013 Mar 21;20(3):403-15. doi: 10.1016/j.chembiol.2012.11.014. Epub 2013 Feb 21. Chem Biol. 2013. PMID: 23434032 Free PMC article. - GroEL/ES chaperonin modulates the mechanism and accelerates the rate of TIM-barrel domain folding.
Georgescauld F, Popova K, Gupta AJ, Bracher A, Engen JR, Hayer-Hartl M, Hartl FU. Georgescauld F, et al. Cell. 2014 May 8;157(4):922-934. doi: 10.1016/j.cell.2014.03.038. Cell. 2014. PMID: 24813614 Free PMC article. - GroEL actively stimulates folding of the endogenous substrate protein PepQ.
Weaver J, Jiang M, Roth A, Puchalla J, Zhang J, Rye HS. Weaver J, et al. Nat Commun. 2017 Jun 30;8:15934. doi: 10.1038/ncomms15934. Nat Commun. 2017. PMID: 28665408 Free PMC article. - Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis.
Ong DS, Mu TW, Palmer AE, Kelly JW. Ong DS, et al. Nat Chem Biol. 2010 Jun;6(6):424-32. doi: 10.1038/nchembio.368. Epub 2010 May 9. Nat Chem Biol. 2010. PMID: 20453863 Free PMC article.
References
- Baumketner A, Jewett A, Shea JE (2003) Effects of confinement in chaperonin assisted protein folding: rate enhancement by decreasing the roughness of the folding energy landscape. J Mol Biol 332: 701–713 - PubMed
- Brinker A, Pfeifer G, Kerner MJ, Naylor DJ, Hartl FU, Hayer-Hartl M (2001) Dual function of protein confinement in chaperonin-assisted protein folding. Cell 107: 223–233 - PubMed
- Castanié HP, Berges H, Oreglia J, Prère MF, Fayet O (1997) A set of pBR322-compatible plasmids allowing the testing of chaperone-assisted folding of proteins overexpressed in Escherichia coli. Anal Biochem 254: 150–152 - PubMed
- Chang HC, Kaiser CM, Hartl FU, Barral JM (2005) De novo folding of GFP fusion proteins: high efficiency in eukaryotes but not in bacteria. J Mol Biol 353: 397–409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials