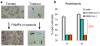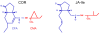Role of stomata in plant innate immunity and foliar bacterial diseases - PubMed (original) (raw)
Review
Role of stomata in plant innate immunity and foliar bacterial diseases
Maeli Melotto et al. Annu Rev Phytopathol. 2008.
Abstract
Pathogen entry into host tissue is a critical first step in causing infection. For foliar bacterial plant pathogens, natural surface openings, such as stomata, are important entry sites. Historically, these surface openings have been considered as passive portals of entry for plant pathogenic bacteria. However, recent studies have shown that stomata can play an active role in limiting bacterial invasion as part of the plant innate immune system. As a counter-defense, the plant pathogen Pseudomonas syringae pv. tomato DC3000 uses the virulence factor coronatine to actively open stomata. In nature, many foliar bacterial disease outbreaks require high humidity, rain, or storms, which could favor stomatal opening and/or bypass stomatal defense by creating wounds as alternative entry sites. Further studies on microbial and environmental regulation of stomatal closure and opening could fill gaps in our understanding of bacterial pathogenesis, disease epidemiology, and microbiology of the phyllosphere.
Figures
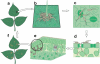
Figure 1
A simplified diagram of the infection cycle of Pseudomonas syringae. (a) A diagram of healthy plant leaves. (b) Bacterial cells on a leaf surface, illustrating aggregation of some bacteria near a trichome. (c) Bacteria penetrating open stomate. (d) Cross section of a leaf showing bacteria colonizing the plant apoplast. (e) Extensive multiplication of bacteria in the apoplast of a leaf. (f) Visible disease-associated necrosis and chlorosis.
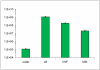
Figure 2
Pst DC3000 populations 8 h after transferring 1.2×106 CFU/ml bacteria in water, Luria-Bertani broth (LB), intercellular wash fluid (IWF), and _hrp_-inducing minimal medium (MM). Data points are means and standard deviations of three replicate cultures.
Figure 3
(a) Light-conditioned leaf epidermis (top pictures) showing mostly open stomata and the same leaf epidermis after 1 h exposure to purified PAMP or live bacteria (bottom pictures). Note that most stomatal pores are closed after exposure to bacteria or PAMPs. (b) Stomatal response in light-exposed epidermal peels of Arabidopsis leaves exposed to water, DC3000, or DB29. DB29 is a coronatine-biosynthesis-defective (cor) mutant (17). Stomatal response to this cor mutant is similar to response to DC3118, another cor mutant (74, 77).
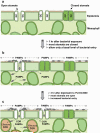
Figure 4
A diagram depicting stomata as entry sites for bacterial invasion. (a) A cross-sectional view of leaf epidermis and mesophyll cells showing that stomata, formed by pairs of guard cells (GC), in light-adapted Arabidopsis leaves are mostly fully open. (b) Upon exposure to bacteria, guard cells perceive PAMPs and many stomata close within 1 h. Because not all stomata are closed, those ‘non-responsive’ open stomata (e.g., stomate on the left) may provide a route for bacterial entry at a basal level. Dashed arrows indicate diffusion of PAMPs on the epidermal surface or in the intercellular space (apoplast) between mesophyll cells, which can also perceive PAMPs and activate additional host defenses. (c) In the case of the virulent plant pathogen Pst DC3000, 3 h after infection bacteria produce diffusible COR in the apoplast and/or on the plant surface to re-open closed stomata (e.g., middle stomate), thereby increasing the number of sites for bacterial invasion. Pst DC3000 bacteria also inject TTSS effectors into mesophyll cells to suppress host defenses and to release nutrients. COR and TTSS effectors have overlapping functions in the suppression of host defenses in the apoplast and in the promotion of disease symptoms (see text). COR also induces disease susceptibility systemically in leaves (; see text). This figure is adapted from Underwood et al. (110) with permission of the Publisher.
Figure 5
Tomato leaves 5 days after dip-inoculation with 1×107 CFU/ml suspension of the wild-type bacterium Pst DC3000 (left) and the coronatine-defective mutant Pst DC3118 (right). Whereas Pst DC3000 caused necrotic lesions with diffuse chlorosis, the coronatine-defective mutant bacteria caused only some lesions and no chlorosis.
Figure 6
The chemical structures of JA-Ile and coronatine are similar.
Figure 7
A model of COR action in the plant cell (possibly all cell types). COR (structure shown) is secreted by Pst DC3000 into the plant cell and increases the affinity of the COI1 protein (as part of the SCFCOI1-ubiquitin ligase complex; not shown here) toward JAZ repressor and possibly other host proteins (denoted by “X”). The SCFCOI1 complex catalyzes ubiquitination of JAZ and other host proteins, which are then degraded through the 26S proteasome (denoted as “26S”). JAZ proteins normally function as repressors by physically binding to transcriptional activators (such as MYC2) of jasmonate response genes. In the stomatal guard cell, PAMPs (e.g., flagellin and LPS) are perceived by cognate immune receptors (e.g., flagellin receptor FLS2). Perception of PAMPs triggers stomatal closure, which requires SA, ABA, and OST1 kinase. COR-mediated inhibition of PAMP-triggered stomatal closure and other innate immune responses could be mediated by JAZ and/or “X” proteins.
Similar articles
- Role of plant stomata in bacterial invasion.
Underwood W, Melotto M, He SY. Underwood W, et al. Cell Microbiol. 2007 Jul;9(7):1621-9. doi: 10.1111/j.1462-5822.2007.00938.x. Epub 2007 Apr 5. Cell Microbiol. 2007. PMID: 17419713 Review. - Stomate-based defense and environmental cues.
Panchal S, Melotto M. Panchal S, et al. Plant Signal Behav. 2017 Sep 2;12(9):e1362517. doi: 10.1080/15592324.2017.1362517. Epub 2017 Aug 17. Plant Signal Behav. 2017. PMID: 28816601 Free PMC article. - Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants.
Xin XF, He SY. Xin XF, et al. Annu Rev Phytopathol. 2013;51:473-98. doi: 10.1146/annurev-phyto-082712-102321. Epub 2013 May 31. Annu Rev Phytopathol. 2013. PMID: 23725467 Review. - A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000.
Zeng W, Brutus A, Kremer JM, Withers JC, Gao X, Jones AD, He SY. Zeng W, et al. PLoS Pathog. 2011 Oct;7(10):e1002291. doi: 10.1371/journal.ppat.1002291. Epub 2011 Oct 6. PLoS Pathog. 2011. PMID: 21998587 Free PMC article. - Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata.
Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF, Bais HP. Kumar AS, et al. Plant J. 2012 Nov;72(4):694-706. doi: 10.1111/j.1365-313X.2012.05116.x. Epub 2012 Sep 24. Plant J. 2012. PMID: 22862801
Cited by
- Early changes in apoplast composition associated with defence and disease in interactions between Phaseolus vulgaris and the halo blight pathogen Pseudomonas syringae Pv. phaseolicola.
O'Leary BM, Neale HC, Geilfus CM, Jackson RW, Arnold DL, Preston GM. O'Leary BM, et al. Plant Cell Environ. 2016 Oct;39(10):2172-84. doi: 10.1111/pce.12770. Epub 2016 Jul 25. Plant Cell Environ. 2016. PMID: 27239727 Free PMC article. - Potential Role of Photosynthesis in the Regulation of Reactive Oxygen Species and Defence Responses to Blumeria graminis f. sp. tritici in Wheat.
Hu Y, Zhong S, Zhang M, Liang Y, Gong G, Chang X, Tan F, Yang H, Qiu X, Luo L, Luo P. Hu Y, et al. Int J Mol Sci. 2020 Aug 11;21(16):5767. doi: 10.3390/ijms21165767. Int J Mol Sci. 2020. PMID: 32796723 Free PMC article. - Expression of extra-cellular levansucrase in Pseudomonas syringae is controlled by the in planta fitness-promoting metabolic repressor HexR.
Mehmood A, Abdallah K, Khandekar S, Zhurina D, Srivastava A, Al-Karablieh N, Alfaro-Espinoza G, Pletzer D, Ullrich MS. Mehmood A, et al. BMC Microbiol. 2015 Feb 26;15:48. doi: 10.1186/s12866-015-0349-0. BMC Microbiol. 2015. PMID: 25886911 Free PMC article. - Hormone interactions in stomatal function.
Acharya BR, Assmann SM. Acharya BR, et al. Plant Mol Biol. 2009 Mar;69(4):451-62. doi: 10.1007/s11103-008-9427-0. Epub 2008 Nov 25. Plant Mol Biol. 2009. PMID: 19031047 Review. - Detection of Bacterial Infection in Melon Plants by Classification Methods Based on Imaging Data.
Pineda M, Pérez-Bueno ML, Barón M. Pineda M, et al. Front Plant Sci. 2018 Feb 14;9:164. doi: 10.3389/fpls.2018.00164. eCollection 2018. Front Plant Sci. 2018. PMID: 29491881 Free PMC article.
References
- Alfano JR, Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 2004;42:385–414. - PubMed
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, et al. MAP kinase signaling in Arabidopsis innate immunity. Nature. 2002;415:977–83. - PubMed
- Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 2005;6:973–79. - PubMed
- Bayot RG, Ries SM. Role of motility in apple blossom infection by Erwinia amylovora and studies of fire blight control with attractant and repellent compounds. Phytopathology. 1986;76:41–45.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials