Epidermal sensing of oxygen is essential for systemic hypoxic response - PubMed (original) (raw)
. 2008 Apr 18;133(2):223-34.
doi: 10.1016/j.cell.2008.02.038.
Alexander Weidemann, Zhenxing Fu, Lernik Mesropian, Katarina Gradin, Colin Jamora, Michael Wiesener, Kai-Uwe Eckardt, Cameron J Koch, Lesley G Ellies, Gabriel Haddad, Volker H Haase, M Celeste Simon, Lorenz Poellinger, Frank L Powell, Randall S Johnson
Affiliations
- PMID: 18423195
- PMCID: PMC2849644
- DOI: 10.1016/j.cell.2008.02.038
Epidermal sensing of oxygen is essential for systemic hypoxic response
Adam T Boutin et al. Cell. 2008.
Abstract
Skin plays an essential role, mediated in part by its remarkable vascular plasticity, in adaptation to environmental stimuli. Certain vertebrates, such as amphibians, respond to hypoxia in part through the skin; but it is unknown whether this tissue can influence mammalian systemic adaptation to low oxygen levels. We have found that epidermal deletion of the hypoxia-responsive transcription factor HIF-1alpha inhibits renal erythropoietin (EPO) synthesis in response to hypoxia. Conversely, mice with an epidermal deletion of the von Hippel-Lindau (VHL) factor, a negative regulator of HIF, have increased EPO synthesis and polycythemia. We show that nitric oxide release induced by the HIF pathway acts on cutaneous vascular flow to increase systemic erythropoietin expression. These results demonstrate that in mice the skin is a critical mediator of systemic responses to environmental oxygen.
Figures
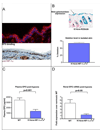
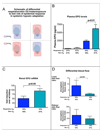
Figure 1. Loss of HIF-1α in the epidermis diminishes renal EPO production during hypoxia
A, The normal mouse epidermis is hypoxic, based on EF5 binding (red), and expresses HIF-1α protein (red). B, K14 cre recombinase transgene expression was verified by β-galactosidase expression in epidermis in the ROSA25 reporter strain; deletion efficiency of the loxP-flanked allele in the epidermis and hair follicle was 70%. C, EPO protein in the plasma following hypoxic exposure for 14 hours at 9% oxygen is significantly reduced in mice lacking HIF-1α in the epidermis (wt n = 25, K14cre-HIF-1α+f/+f n = 11). D, Renal EPO mRNA expression is reduced to normoxic levels in hypoxic K14cre-HIF-1α+f/+f mice (wt n = 6, K14cre-HIF-1α+f/+f n = 3). All graphs represent mean ± SEM.
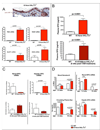
Figure 2. Deletion of VHL in the epidermis induces EPO production during normoxia
A, Upon deletion of VHL in the epidermis, HIF-1α protein (red) is stabilized and HIF target gene expression is increased in the skin (for each graph wt n = 3, K14cre-VHL+f/+f n = 3, for RNA isolation). B, Constitutive or Tamoxifen-induced epidermal deletion of VHL results in highly elevated plasma EPO (wt n = 36, K14cre-VHL+f/+f n = 23, K14TAMcre-VHL+f/+f n = 5). C, In the K14cre-VHL+f/+f mouse, EPO mRNA expression is suppressed in the kidney but increased in the liver. In the tamoxifen inducible K14TAMcre-VHL+f/+f, where deletion occurs in the adult, EPO expression is increased in the kidney, and unaffected in the liver (wt n = 9, K14cre-VHL+f/+f n = 6, K14TAMcre-VHL+f/+f n = 5). D, As blood hematocrit levels increase in the constitutively deleted K14cre-VHL+f/+f mice (wt n = 43, K14cre-VHL+f/+f 1.5 wks n = 3, K14cre-VHL+f/+f 10 wks n = 32), renal EPO mRNA expression is suppressed and hepatic EPO increases, indicating that hematocrit can selectively affect renal EPO expression (wt n = 9, K14cre-VHL+f/+f 1.5 wks n = 3, K14cre-VHL+f/+f 10 wks n = 6). All graphs represent mean ± SEM.
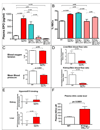
Figure 3. K14cre-VHL+f/+f demonstrates altered blood flow, increased internal hypoxia, and increased EPO expression. Restoration to wild type levels by co-deletion of HIF-2α
A, Deletion of HIF-2α but not HIF-1α in the K14cre-VHL+f/+f background restores plasma EPO (wt n = 36, K14cre-VHL+f/+f n = 23, K14cre-VHL+f/+fHIF-1α+f/+f n = 15, K14cre-VHL+f/+f HIF-2α+f/+f n = 3, K14cre-VHL+f/+f HIF-1α+f/+f f HIF-2α+f/+f n = 3) and blood hematocrit (B) to wild type levels (wt n = 43, K14cre-VHL+f/+f n = 32, K14cre-VHL+f/+f HIF-1α+f/+f n = 17, K14cre-VHL+f/+f HIF-2α+f/+f n = 3, K14cre-VHL+f/+f HIF-1α+f/+f f HIF-2α+f/+f n = 3). Deletion of both HIF-1α and HIF-2α is similar in effect to deletion of HIF-2α alone. C, Blood oxygen saturation is normal in K14cre-VHL+f/+f animals (wt n = 7, K14cre-VHL+f/+f n = 6), but animals are hypotensive (wt n = 17, K14cre-VHL+f/+f n = 10). D, Blood flow in the K14cre-VHL+f/+f is shifted away from the liver and kidney, and toward the skin, as measured by microsphere distribution (wt n = 11, K14cre-VHL+f/+f n = 4). E, The shift in blood flow corresponds to increased EF5 binding/hypoxia in the kidney and liver of K14cre-VHL+f/+f (wt n = 8, K14cre-VHL+f/+f n = 4). F, Nitric oxide metabolites are increased in K14cre-VHL+f/+f plasma, demonstrating increased NO production (wt n = 33, K14cre-VHL+f/+f n = 8). All graphs represent mean ± SEM.
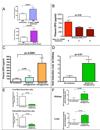
Figure 4. Nitric Oxide production in the skin mediates shift in blood flow and renal EPO expression in K14cre-VHL+f/+f mice
A, Systemic inhibition of NO synthesis by L-NAME increases plasma EPO in wild type mice (wt n = 36, wt + L-NAME n = 12). Global iNOS knockout mice show significantly elevated EPO plasma levels following hypoxia relative to wild type mice (wt n = 25, iNOS−/− n = 7). B, NO synthesis inhibition by treatment with L-NAME restores plasma EPO levels to levels similar to L-NAME-treated wild type mice, when administered to K14cre-VHL+f/+f mice (K14cre-VHL+f/+f n = 23, K14cre-VHL+f/+f + L-NAME 3 days n = 3, K14cre-VHL+f/+f + L-NAME 4 days n = 4). C, NO donor (nitroglycerine) applied to the skin of wild type mice increases plasma EPO levels; similar systemic doses of NO donor do not significantly affect plasma EPO levels (control n = 20, Systemic nitroglycerine n = 3, Epidermal nitroglycerine n = 9). D, Epidermal NO donor administration induces renal EPO mRNA expression (control n = 9, Epidermal nitroglycerine n = 3). E, Epidermal nitroglycerin shifts blood flow (control n = 11, Epidermal nitroglycerine n = 6) towards the skin and increases renal and hepatic hypoxia (F), in a manner similar to that seen in K14cre-VHL+f/+f mice (control n = 8, Epidermal nitroglycerine n = 5). All graphs represent mean ± SEM.
Figure 5. Skin hypoxia directly affects overall systemic hypoxic response in wild type mice exposed to acute hypoxia
A, Special chambers were constructed to isolate inhaled oxygen concentration from the oxygen concentration exposed to the skin. B, In mice breathing 21% O2, skin hypoxia was not enough to induce increased EPO synthesis. However, in mice breathing 10% O2, plasma EPO levels were significantly elevated when skin was normoxic (Respired 21% O2 Skin 21% O2 n = 2, Respired 21% O2 Skin 10% O2 n = 3, Respired 10% O2 Skin 10% O2 n = 8, Respired 10% O2 Skin 21% O2 n = 9); mice exposed to differential gases for 5 hours. C, Renal EPO expression was higher in mice breathing 10% O2 while exposed to 21% O2 (Respired 10% O2 Skin 10% O2 n = 6, Respired 10% O2 Skin 21% O2 n = 7); mice exposed to differential gases for 5 hours. D, Blood flow is shifted from the skin to the kidney and liver when the skin is hypoxic for 1 hour. This shift is absent when the skin is exposed to normoxia (Respired 10% O2 Skin 10% O2 n = 7, Respired 10% O2 Skin 21% O2 n = 8). All graphs represent mean ± SEM.
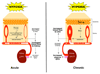
Figure 6. Acute and chronic adaptation to hypoxia is influenced by dermal response
Hypoxia may act through mechanisms similar to those found in the lung to induce pulmonary vasoconstrictions under acute hypoxic stress; a more chronic stress allows a HIF-induced modulation of response that also affects blood flow and EPO expression.
Comment in
- O2 sensing: only skin deep?
Semenza GL. Semenza GL. Cell. 2008 Apr 18;133(2):206-8. doi: 10.1016/j.cell.2008.04.004. Cell. 2008. PMID: 18423190
Similar articles
- Action of hypoxia-inducible factor in liver and kidney from mice with Pax8-rtTA-based deletion of von Hippel-Lindau protein.
Mathia S, Paliege A, Koesters R, Peters H, Neumayer HH, Bachmann S, Rosenberger C. Mathia S, et al. Acta Physiol (Oxf). 2013 Mar;207(3):565-76. doi: 10.1111/apha.12058. Acta Physiol (Oxf). 2013. PMID: 23384425 - The Mitochondrial Respiratory Chain Is Required for Organismal Adaptation to Hypoxia.
Hamanaka RB, Weinberg SE, Reczek CR, Chandel NS. Hamanaka RB, et al. Cell Rep. 2016 Apr 19;15(3):451-459. doi: 10.1016/j.celrep.2016.03.044. Epub 2016 Apr 7. Cell Rep. 2016. PMID: 27068470 Free PMC article. - The VHL/HIF oxygen-sensing pathway and its relevance to kidney disease.
Haase VH. Haase VH. Kidney Int. 2006 Apr;69(8):1302-7. doi: 10.1038/sj.ki.5000221. Kidney Int. 2006. PMID: 16531988 Review. - The HIF pathway and erythrocytosis.
Lee FS, Percy MJ. Lee FS, et al. Annu Rev Pathol. 2011;6:165-92. doi: 10.1146/annurev-pathol-011110-130321. Annu Rev Pathol. 2011. PMID: 20939709 Review.
Cited by
- Hypoxia-Inducible Factor Signaling in Macrophages Promotes Lymphangiogenesis in Leishmania major Infection.
Bowlin A, Roys H, Wanjala H, Bettadapura M, Venugopal G, Surma J, Simon MC, Weinkopff T. Bowlin A, et al. Infect Immun. 2021 Jul 15;89(8):e0012421. doi: 10.1128/IAI.00124-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 34031127 Free PMC article. - Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function.
McNamee EN, Korns Johnson D, Homann D, Clambey ET. McNamee EN, et al. Immunol Res. 2013 Mar;55(1-3):58-70. doi: 10.1007/s12026-012-8349-8. Immunol Res. 2013. PMID: 22961658 Free PMC article. Review. - Anaemia in kidney disease: harnessing hypoxia responses for therapy.
Koury MJ, Haase VH. Koury MJ, et al. Nat Rev Nephrol. 2015 Jul;11(7):394-410. doi: 10.1038/nrneph.2015.82. Epub 2015 Jun 9. Nat Rev Nephrol. 2015. PMID: 26055355 Free PMC article. Review. - The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism.
Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F, Johnson RS. Zhang N, et al. Cell Metab. 2010 May 5;11(5):364-78. doi: 10.1016/j.cmet.2010.03.001. Epub 2010 Apr 15. Cell Metab. 2010. PMID: 20399150 Free PMC article. - Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway.
Sondermann NC, Faßbender S, Hartung F, Hätälä AM, Rolfes KM, Vogel CFA, Haarmann-Stemmann T. Sondermann NC, et al. Biochem Pharmacol. 2023 Feb;208:115371. doi: 10.1016/j.bcp.2022.115371. Epub 2022 Dec 15. Biochem Pharmacol. 2023. PMID: 36528068 Free PMC article. Review.
References
- Abbrecht PH, Littell JK. Plasma erythropoietin in men and mice during acclimatization to different altitudes. J Appl Physiol. 1972;32:54–58. - PubMed
- Bergmann C. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien. 1847:595–708.
- Bozzini CE, Barcelo AC, Conti MI, Martinez MP, Alippi RM. Enhanced erythropoietin production during hypobaric hypoxia in mice under treatments to keep the erythrocyte mass from rising: implications for the adaptive role of polycythemia. High Alt Med Biol. 2005;6:238–246. - PubMed
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. - PubMed
- Durand J, Verpillat JM, Pradel M, Martineaud JP. Influence of altitude on the cutaneous circulation of residents and newcomers. Fed Proc. 1969;28:1124–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K22CA118182/CA/NCI NIH HHS/United States
- R01 CA100787/CA/NCI NIH HHS/United States
- R01 CA082515-09/CA/NCI NIH HHS/United States
- R01CA082515/CA/NCI NIH HHS/United States
- R01AI060840/AI/NIAID NIH HHS/United States
- R01 CA082515/CA/NCI NIH HHS/United States
- R01 HL081823/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials