Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor - PubMed (original) (raw)
Peripheral CD8+ T cell tolerance to self-proteins is regulated proximally at the T cell receptor
Ryan M Teague et al. Immunity. 2008 May.
Abstract
CD8(+) T cell tolerance, although essential for preventing autoimmunity, poses substantial obstacles to eliciting immune responses to tumor antigens, which are generally overexpressed normal proteins. Development of effective strategies to overcome tolerance for clinical applications would benefit from elucidation of the immunologic mechanism(s) regulating T cell tolerance to self. To examine how tolerance is maintained in vivo, we engineered dual-T cell receptor (TCR) transgenic mice in which CD8(+) T cells recognize two distinct antigens: a foreign viral-protein and a tolerizing self-tumor protein. Encounter with peripheral self-antigen rendered dual-TCR T cells tolerant to self, but these cells responded normally through the virus-specific TCR. Moreover, proliferation induced by virus rescued function of tolerized self-tumor-reactive TCR, restoring anti-tumor activity. These studies demonstrate that peripheral CD8(+) T cell tolerance to self-proteins can be regulated at the level of the self-reactive TCR complex rather than by central cellular inactivation and suggest an alternate strategy to enhance adoptive T cell immunotherapy.
Figures
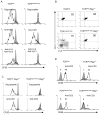
Figure 1. Activation of tolerant CD8+ T cells with anti-CD3
(A) CFSE-dilution in CD8-gated naïve and tolerant splenocytes from TCRGag and TCRGagxAlb:Gag mice stimulated with an irrelevant Env (grey) or the Gag-peptide, anti-CD3, or antibody to Vα3 and Vβ12 (black line) for 3 days in vitro. (B) Splenocytes from Rag1+/+ and Rag1−/− TCRGag and TCRGagxAlb:Gag mice were analyzed for TCR expression by flow cytometry. Dot plots represent CD8-gated cells, and the percentage of Vα3+Vβ12+ T cells within the CD8+ population is indicated (Data representative of >10 mice from each genotype). (C) CFSE-dilution in CD8-gated Rag1−/− TCRGag and TCRGagxAlb:Gag splenocytes stimulated with Env (grey), Gag, or anti-CD3 (black line) for 3 days in vitro. (D) Staining with anti-CD69 (black line) or isotype control antibody (grey), was analyzed on CD4-depleted Vα3+Vβ12+ Rag1+/+ or Rag1−/− TCRGag and TCRGagxAlb:Gag splenocytes 5 hours after stimulation with anti-CD3. The mean fluorescence intensity (MFI) for each peak is provided and data are representative of 4 separate experiments.
Figure 2. Phenotype of single and dual-TCR CD8+ T cells
Splenocytes from TCRGag, P14 and the F1 naive (P14-TCRGag) and tolerant (P14-TCRGagxAlb:Gag) dual-TCR progeny were gated on CD8+ cells and analyzed for expression of (A) Vα3 and Vβ12 (TCRGag), or (B) Vα2 and Vβ8 (P14), or (C) both sets of TCRα chains (Vα2 and Vα3). The percent of double-positive CD8+ cells is inset. (D) Single and dual-receptor CD8+ T cells (circled in 2c) were analyzed for CD44 expression. Inset numbers represent MFI, and data are representative of more than 10 mice from each genotype. (E) Transgenic P14-TCRGag and P14-TCRGagxAlb:Gag splenocytes (Thy1.2) were FACS sorted based on CD44 and CD8 expression (within rectangle regions). Sorted T cells were combined 1:10 with congenic APC from a Thy1.1+ mouse, labeled with CFSE and stimulated with control, Gag or Gp33 peptide for 96 hours in vitro, and CFSE-dilution in Thy1.2+ T cells assessed. The percent of Thy1.2+ cells that had diluted CFSE is indicated. Data are representative of 3 experiments.
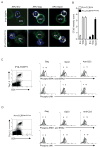
Figure 3. Distinct signaling through tolerant and naïve TCR
(A) P14-TCRGag and P14-TCRGagxAlb:Gag splenocytes were sorted based on CD44 and CD8 expression. CD8+ T cells (blue) were stained for CTxB (yellow to white) after 30 min encounter with peptide-pulsed APC (green) and visualized by confocal microscopy, (B) and graded 0–5 for CTxB staining intensity. Average scores from 3 experiments with P14-TCRGag (open bars) and P14-TCRGagxAlb:Gag (filled bars) cells are presented with standard error of the mean. (C) Sorted naive P14-TCRGag and (D) tolerant P14-TCRGagxAlb:Gag T cells (Thy1.2) were combined 1:10 with APC form a congenic Thy1.1+ mouse, and stimulated with control (grey filled), Gag or Gp33-peptide (black lines) or anti-CD3 (black lines) in vitro. Phosphorylation of ERK and JNK in cells gated for Thy1.2 and CD8 expression (left) was assessed after 30 min by intracellular staining with phospho-specific antibodies. MFI values for each peak are inset, and data are representative of 4 separate experiments.
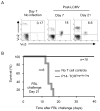
Figure 4. In vivo expansion and rescue of tolerant dual-TCR T cells
Transgenic naive P14-TCRGag and tolerant P14-TCRGagxAlb:Gag splenocytes were FACS sorted based on CD44 and CD8 expression, and 1×105 sorted T cells transferred into B6 or Alb:Gag recipients. Recipient mice were immunized with either (A) LCMV or (B) peptide-pulsed irradiated E10 cells. Inset numbers are percent of total CD8+ cells from PBL at day 7 post-immunization. (C) 1×105 FACS sorted naive P14-TCRGag and tolerant P14-TCRGagxAlb:Gag T cells were transferred into Thy1.1 or Alb:GagThy1.1 recipients and infected with LCMV for 7 days. Recipient splenocytes were stimulated ex vivo with control Env (grey filled), Gag or Gp33-peptide, or anti-CD3 (black lines), and phosphorylation of ERK and JNK in cells gated for CD8 and Thy.1.2 expression (circled in dot plots on the left) assessed after 30 min. The MFI for each peak is inset. (D) Dual-TCR T cells were analyzed for relative TCR expression directly ex vivo from non-infected mice or after CD8 and CD44 FACS sorting and transfer into B6 or Alb:Gag recipients and infection with LCMV for 7 days. Splenocytes were CD4-depleted and expression of Vα3 and Vβ12 assessed on cells gated for Vα2 expression by flow cytometry. Data are representative of 3 separate experiments.
Figure 5. In vivo expansion and anti-FBL effector function by dual-TCR T cells
1×105 sorted naive P14-TCRGag or tolerant P14-TCRGagxAlb:Gag T cells were transferred into B6 or Alb:Gag recipients respectively, and infected with LCMV. (A) At 7, 14, 21 and 40 days after infection, recipient PBL were analyzed for Vα2+Vα3+CD8+ T cells. Error bars are standard deviation of 20 mice per group from 4 separate experiments. Recipients were challenged with live FBL tumor (B) 7 days, (C) 14 days and (D) 21 days after LCMV infection. Survival was monitored for 40 days post-tumor challenge and each graph represents pooled data from the indicated number of mice (n) per group from 2 separate experiments.
Figure 6. In vivo expansion and anti-FBL effector function in the absence of tolerizing peripheral antigen
(A) The frequency of Vα2+Vα3+CD8+ cells in PBL from B6 recipients of 1×105 FACS sorted CD44hi CD8+ tolerant P14-TCRGagxAlb:Gag T cells was assessed at days 7 and 21 after LCMV infection (or no infection control recipients). (B) B6 mice receiving 1×105 sorted tolerant P14-TCRGagxAlb:Gag T cells (black line) or no T cell controls (bold grey line) were challenged with 1×106 live FBL tumor by i.p injection 21 days after LCMV infection. Survival was monitored for 40 days and the graph represents pooled data from the indicated number of mice (n) per experimental group from 2 independent experiments.
Figure 7. Restimulation of contracted and tolerant dual-TCR T cells within the tolerizing environment
(A) Vα2+Vα3+CD8+ cells from Alb:Gag recipients of 1×105 FACS sorted CD44hi CD8+ tolerant P14-TCRGagxAlb:Gag T cells were assessed 30 days after LCMV infection, and 7 days after secondary boost immunization (day 37) with either Gag or Gp33 peptide-pulsed B6 splenocytes. (B) At day 37 (7 days post-boost), mice were challenged with live FBL tumor and survival monitored for 40 days. The graph represents pooled data from the indicated number of mice (n) per experimental group from 3 independent experiments.
Comment in
- Anergic signals: the action is upstream.
Gu H. Gu H. Immunity. 2008 May;28(5):598-600. doi: 10.1016/j.immuni.2008.04.005. Immunity. 2008. PMID: 18482562
Similar articles
- CD8 T cell tolerance to a tumor-associated self-antigen is reversed by CD4 T cells engineered to express the same T cell receptor.
Ghorashian S, Veliça P, Chua I, McNicol AM, Carpenter B, Holler A, Nicholson E, Ahmadi M, Zech M, Xue SA, Uckert W, Morris E, Chakraverty R, Stauss HJ. Ghorashian S, et al. J Immunol. 2015 Feb 1;194(3):1080-9. doi: 10.4049/jimmunol.1401703. Epub 2014 Dec 24. J Immunol. 2015. PMID: 25539815 Free PMC article. - An Essential Role of the Avidity of T-Cell Receptor in Differentiation of Self-Antigen-reactive CD8+ T Cells.
Kondo K, Fujiki F, Nakajima H, Yatsukawa E, Morimoto S, Tatsumi N, Nishida S, Nakata J, Oka Y, Tsuboi A, Hosen N, Oji Y, Sugiyama H. Kondo K, et al. J Immunother. 2016 Apr;39(3):127-39. doi: 10.1097/CJI.0000000000000114. J Immunother. 2016. PMID: 26938946 - CD8+ T cell exhaustion during persistent viral infection is regulated independently of the virus-specific T cell receptor.
Jackson SR, Berrien-Elliott MM, Meyer JM, Wherry EJ, Teague RM. Jackson SR, et al. Immunol Invest. 2013;42(3):204-20. doi: 10.3109/08820139.2012.751397. Epub 2013 Mar 5. Immunol Invest. 2013. PMID: 23461613 Free PMC article. - Narcissistic T cells: reactivity to self makes a difference.
Paprckova D, Stepanek O. Paprckova D, et al. FEBS J. 2021 Mar;288(6):1778-1788. doi: 10.1111/febs.15498. Epub 2020 Aug 10. FEBS J. 2021. PMID: 32738029 Review. - How the TCR balances sensitivity and specificity for the recognition of self and pathogens.
Morris GP, Allen PM. Morris GP, et al. Nat Immunol. 2012 Jan 19;13(2):121-8. doi: 10.1038/ni.2190. Nat Immunol. 2012. PMID: 22261968 Review.
Cited by
- Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance.
Berrien-Elliott MM, Jackson SR, Meyer JM, Rouskey CJ, Nguyen TL, Yagita H, Greenberg PD, DiPaolo RJ, Teague RM. Berrien-Elliott MM, et al. Cancer Res. 2013 Jan 15;73(2):605-16. doi: 10.1158/0008-5472.CAN-12-2179. Epub 2012 Nov 27. Cancer Res. 2013. PMID: 23188506 Free PMC article. - Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity.
Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, Gunset G, Perna F, Kreines FM, Levy ER, Lieberman S, Jay HV, Tuckett AZ, Zakrzewski JL, Tan L, Young LF, Takvorian K, Dudakov JA, Jenq RR, Hanash AM, Motta AC, Murphy GF, Liu C, Schietinger A, Sadelain M, van den Brink MR. Ghosh A, et al. Nat Med. 2017 Feb;23(2):242-249. doi: 10.1038/nm.4258. Epub 2017 Jan 9. Nat Med. 2017. PMID: 28067900 Free PMC article. - Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state.
Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Schietinger A, et al. Science. 2012 Feb 10;335(6069):723-7. doi: 10.1126/science.1214277. Epub 2012 Jan 19. Science. 2012. PMID: 22267581 Free PMC article. - Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper.
Palmer E, Naeher D. Palmer E, et al. Nat Rev Immunol. 2009 Mar;9(3):207-13. doi: 10.1038/nri2469. Nat Rev Immunol. 2009. PMID: 19151748 Review. - Inflammation programs self-reactive CD8+ T cells to acquire T-box-mediated effector function but does not prevent deletional tolerance.
Jackson SR, Yuan J, Berrien-Elliott MM, Chen CL, Meyer JM, Donlin MJ, Teague RM. Jackson SR, et al. J Leukoc Biol. 2014 Sep;96(3):397-410. doi: 10.1189/jlb.1A0913-500RR. Epub 2014 May 13. J Leukoc Biol. 2014. PMID: 24823810 Free PMC article.
References
- Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8+ T-cell memory. Nat Rev Immunol. 2005;5:101–111. - PubMed
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. - PubMed
- Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–1383. - PMC - PubMed
- Cheng LE, Greenberg PD. Selective delivery of augmented IL-2 receptor signals to responding CD8+ T cells increases the size of the acute antiviral response and of the resulting memory T cell pool. J Immunol. 2002;169:4990–4997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA33084/CA/NCI NIH HHS/United States
- CA18029/CA/NCI NIH HHS/United States
- P01 CA018029/CA/NCI NIH HHS/United States
- R37 CA033084/CA/NCI NIH HHS/United States
- R01 CA033084/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials