Iron-dependent regulation of frataxin expression: implications for treatment of Friedreich ataxia - PubMed (original) (raw)
. 2008 Aug 1;17(15):2265-73.
doi: 10.1093/hmg/ddn127. Epub 2008 Apr 17.
Affiliations
- PMID: 18424449
- PMCID: PMC2465796
- DOI: 10.1093/hmg/ddn127
Iron-dependent regulation of frataxin expression: implications for treatment of Friedreich ataxia
Kuanyu Li et al. Hum Mol Genet. 2008.
Abstract
Friedreich ataxia (FA) is a progressive neurodegenerative disease caused by expansion of a trinucleotide repeat within the first intron of the gene that encodes frataxin. In our study, we investigated the regulation of frataxin expression by iron and demonstrated that frataxin mRNA levels decrease significantly in multiple human cell lines treated with the iron chelator, desferal (DFO). In addition, frataxin mRNA and protein levels decrease in fibroblast and lymphoblast cells derived from both normal controls and from patients with FA when treated with DFO. Lymphoblasts and fibroblasts of FA patients have evidence of cytosolic iron depletion, as indicated by increased levels of iron regulatory protein 2 (IRP2) and/or increased IRE-binding activity of IRP1. We postulate that this inferred cytosolic iron depletion occurs as frataxin-deficient cells overload their mitochondria with iron, a downstream regulatory effect that has been observed previously when mitochondrial iron-sulfur cluster assembly is disrupted. The mitochondrial iron overload and presumed cytosolic iron depletion potentially further compromise function in frataxin-deficient cells by decreasing frataxin expression. Thus, our results imply that therapeutic efforts should focus on an approach that combines iron removal from mitochondria with a treatment that increases cytosolic iron levels to maximize residual frataxin expression in FA patients.
Figures
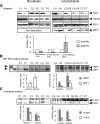
Figure 1.
Frataxin (FXN) mRNA expression is regulated by iron status in human cell lines. HEK293 cells were treated with desferal (DFO, 100 µM), normal unsupplemented medium (Ctr) or ferric ammonium citrate (FAC, 200 µM) for 16 h. (A) mRNA levels of FXN decreased in DFO treated cells compared with control (Ctr) and FAC treated cells. Equal loading was verified by GAPDH and L32 measurements. (B) Quantitative real-time PCR (qRT–PCR) was carried out for FXN and mRNA levels decreased significantly (P = 0.0013) after DFO treatment. (C) Northern analysis and (D) qRT–PCR were carried out for FXN on fibroblasts (GM08399) derived from a normal control, treated either with DFO (100 µM) or FAC (200 µM). Frataxin levels in qRT–PCR were significantly reduced (P = 0.028). Ribosomal RNA levels (28S and 18S) were used as loading controls for (C) and GAPDH was used as an internal control for (D). (E) Luciferase activity driven by FXN promoter decreased significantly (P = 0.0025) when the cells were treated with DFO compared with growth in normal iron-replete media. The empty plasmid was used as a negative control. FXN-luc1, 2 and 3 contained different fragments of genomic DNA, and FXN-GAA-luc contained the frataxin promoter, first exon and a portion of the first intron as described in Materials and Methods. Statistical analysis was done with the Student's _t_-test: *P < 0.05, **P < 0.01.
Figure 2.
Half-life determination of human FXN in HEK293 cells with metabolic labeling. (A) Endogenous FXN of HEK293 cells was chased and only the precursor (p) and intermediate (i) forms were identified. (B) and (C) A mutagenized form of frataxin (FXN L200/203M- described in Materials and Methods) was expressed in HEK293 cells to allow visualization of the mature isoform (m). The half life of human FXN was calculated from three independent experiments, and precursor (p), intermediate (i) and mature (m) forms are represented in lower panels of (B and C) with the half-life of the mature form calculated as ∼50 h. (D) The amino acid sequence of the human FXN open reading frame. The initiator methionine and an in-frame methionine (M) are underlined. The inverted arrowheads indicate proposed sites of cleavage associated with mitochondrial uptake. The first two cleavage sites were initially proposed according to molecular weight in a number of in vitro studies (19,43), whereas the third cleavage site of human endogenous FXN was identified by mass spectrometry (18). The last two leucines (L) were replaced with methionine (M), underlined and in italics.
Figure 3.
Iron deprivation reduces FXN protein levels. Northern analysis (A) and qRT–PCR (B) were performed for FXN in fibroblasts GM03665 derived from an FA patient. Ribosomal RNAs (28S and 18S) were used as loading controls in (A) and GAPDH was used to normalize in (B), where frataxin levels were significantly reduced by treatment with desferal (**P = 0.0006). (C) Immunoblots showed that FXN protein levels diminished with iron deprivation, observed with DFO treatment in the rhabdomyosarcoma cell line RD4 (top), HEK293 cells (middle) and lymphoblasts from an FA patient (GM15850) (bottom). Transferrin receptor (TfR) and ferritin (H- and L subunits, H-Ft and L-Ft) were used to verify that iron treatments were efficacious. (D) Biosynthetic labeling of FXN was performed after cellular iron status was manipulated. HEK293 cells were treated with DFO (30 µM) or FAC (200 µM) for 48 h prior to labeling with [35S]-methionine at the indicated time points. Immunoprecipitation was performed using equal counts of radioactively labeled material.
Figure 4.
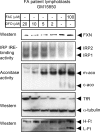
Cell lines derived from FA patients show evidence of cytosolic iron depletion when grown under normal conditions. Either fibroblasts (C1: GM08402 and P1:GM04078; C2:AG09429 and P2:GM03816; C3: GM08399 and P3: GM03665, left column) or lymphoblasts (C4: GM15849 and P4:GM15850, C5: GM16200 and P5:GM16197, C6: GM16215 and P6:GM16214, C7: AG15799 and P7:GM16209, right column) derived from both healthy controls (C) and FA patients (P) were compared. Representative results from multiple repetitions are shown here. Numbers 1–7 for controls and patients represent either age and sex-matched (C1/P1, C2/P2, C3/P3, C4/P4, C7/P7) pairs or proband and parent-paired (C5/P5, C6/P6) pairs. (A) The relative protein levels of FXN, IRP1 and IRP2 were determined by western blot, quantified as described in Materials and Methods. Both IRE-binding activities of IRP1 (white bar) and IRP2 (grey bar) (B) and aconitase activities (C) (mitochondrial, m-aco, grey bar; cytosolic, c-aco, white bar) were assessed and quantified, showing significant decreases in aconitase activities (*P < 0.05), and increases in IRE binding activities (**P < 0.01).
Figure 5.
A titration curve of FA patient cells reveals that FA cells are markedly affected by iron depletion, which further reduces FXN expression. Patient lymphoblasts GM15850 were treated either with indicated concentrations of DFO or with FAC for 60 h, then harvested, lysed and analyzed by the in-gel aconitase assay, bandshift assay and western blot. The abbreviations are the same as those used in Fig. 4.
Figure 6.
A model of how cytosolic iron depletion decreases expression of frataxin in FA patients and may thereby exacerbate disease. Frataxin deficiency mainly caused by the triplet GAA repeat expansion in the first intron of frataxin gene leads to decreased frataxin expression and impairment of mitochondrial iron–sulfur cluster [Fe–S] assembly. Mitochondrial iron overload develops as a consequence of deficient [Fe–S] assembly in mitochondria which leads to misregulation of mitochondrial iron homeostasis. Excess mitochondrial iron uptake and sequestration causes cytosolic iron depletion, which can further diminish frataxin expression by decreasing frataxin transcription. Thus, the decrease in frataxin levels caused by the trinucleotide repeat may be worsened as cells accumulate mitochondrial iron and deplete cytosolic iron stores. This negative feedback loop may contribute to progression of disease in long-lived cells, particularly neurons and cardiomyocytes.
Similar articles
- Exenatide induces frataxin expression and improves mitochondrial function in Friedreich ataxia.
Igoillo-Esteve M, Oliveira AF, Cosentino C, Fantuzzi F, Demarez C, Toivonen S, Hu A, Chintawar S, Lopes M, Pachera N, Cai Y, Abdulkarim B, Rai M, Marselli L, Marchetti P, Tariq M, Jonas JC, Boscolo M, Pandolfo M, Eizirik DL, Cnop M. Igoillo-Esteve M, et al. JCI Insight. 2020 Jan 30;5(2):e134221. doi: 10.1172/jci.insight.134221. JCI Insight. 2020. PMID: 31877117 Free PMC article. - Peptide SS-31 upregulates frataxin expression and improves the quality of mitochondria: implications in the treatment of Friedreich ataxia.
Zhao H, Li H, Hao S, Chen J, Wu J, Song C, Zhang M, Qiao T, Li K. Zhao H, et al. Sci Rep. 2017 Aug 29;7(1):9840. doi: 10.1038/s41598-017-10320-2. Sci Rep. 2017. PMID: 28852135 Free PMC article. - Normal and Friedreich ataxia cells express different isoforms of frataxin with complementary roles in iron-sulfur cluster assembly.
Gakh O, Bedekovics T, Duncan SF, Smith DY 4th, Berkholz DS, Isaya G. Gakh O, et al. J Biol Chem. 2010 Dec 3;285(49):38486-501. doi: 10.1074/jbc.M110.145144. Epub 2010 Oct 2. J Biol Chem. 2010. PMID: 20889968 Free PMC article. - Therapeutic developments in Friedreich ataxia.
Wilson RB. Wilson RB. J Child Neurol. 2012 Sep;27(9):1212-6. doi: 10.1177/0883073812449691. Epub 2012 Jul 12. J Child Neurol. 2012. PMID: 22791549 Free PMC article. Review. - Iron metabolism and mitochondrial abnormalities in Friedreich ataxia.
Pandolfo M. Pandolfo M. Blood Cells Mol Dis. 2002 Nov-Dec;29(3):536-47; discussion 548-52. doi: 10.1006/bcmd.2002.0591. Blood Cells Mol Dis. 2002. PMID: 12547248 Review.
Cited by
- Estrogen prevents oxidative damage to the mitochondria in Friedreich's ataxia skin fibroblasts.
Richardson TE, Yu AE, Wen Y, Yang SH, Simpkins JW. Richardson TE, et al. PLoS One. 2012;7(4):e34600. doi: 10.1371/journal.pone.0034600. Epub 2012 Apr 3. PLoS One. 2012. PMID: 22509330 Free PMC article. - Therapeutic strategies in Friedreich's ataxia.
Richardson TE, Kelly HN, Yu AE, Simpkins JW. Richardson TE, et al. Brain Res. 2013 Jun 13;1514:91-7. doi: 10.1016/j.brainres.2013.04.005. Epub 2013 Apr 13. Brain Res. 2013. PMID: 23587934 Free PMC article. Review. - Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts.
Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, Rouault TA. Ye H, et al. J Clin Invest. 2010 May;120(5):1749-61. doi: 10.1172/JCI40372. Epub 2010 Apr 1. J Clin Invest. 2010. PMID: 20364084 Free PMC article. - Diagnosis and treatment of Friedreich ataxia: a European perspective.
Schulz JB, Boesch S, Bürk K, Dürr A, Giunti P, Mariotti C, Pousset F, Schöls L, Vankan P, Pandolfo M. Schulz JB, et al. Nat Rev Neurol. 2009 Apr;5(4):222-34. doi: 10.1038/nrneurol.2009.26. Nat Rev Neurol. 2009. PMID: 19347027 Review. - Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases.
Zhou ZD, Tan EK. Zhou ZD, et al. Mol Neurodegener. 2017 Oct 23;12(1):75. doi: 10.1186/s13024-017-0218-4. Mol Neurodegener. 2017. PMID: 29061112 Free PMC article. Review.
References
- Pandolfo M. Iron and Friedreich ataxia. J. Neural. Transm. Suppl. 2006:143–146. - PubMed
- Puccio H., Simon D., Cossee M., Criqui-Filipe P., Tiziano F., Melki J., Hindelang C., Matyas R., Rustin P., Koenig M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 2001;27:181–186. - PubMed
- Koeppen A.H., Michael S.C., Knutson M.D., Haile D.J., Qian J., Levi S., Santambrogio P., Garrick M.D., Lamarche J.B. The dentate nucleus in Friedreich's ataxia: the role of iron-responsive proteins. Acta. Neuropathol. 2007;114:163–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials