Up on the tightrope: natural killer cell activation and inhibition - PubMed (original) (raw)
Review
Up on the tightrope: natural killer cell activation and inhibition
Lewis L Lanier. Nat Immunol. 2008 May.
Abstract
Natural killer (NK) cells circulate through the blood, lymphatics and tissues, on patrol for the presence of transformed or pathogen-infected cells. As almost all NK cell receptors bind to host-encoded ligands, signals are constantly being transmitted into NK cells, whether they interact with normal or abnormal cells. The sophisticated repertoire of activating and inhibitory receptors that has evolved to regulate NK cell activity ensures that NK cells protect hosts against pathogens, yet prevents deleterious NK cell-driven autoimmune responses. Here I highlight recent advances in our understanding of the structural properties and signaling pathways of the inhibitory and activating NK cell receptors, with a particular focus on the ITAM-dependent activating receptors, the NKG2D-DAP10 receptor complexes and the CD244 receptor system.
Figures
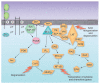
Figure 1
ITAM-containing NK receptors. Schematic representation of NK receptors of the immunoglobulin superfamily or C-type lectin—like family that pair with the ITAM-bearing DAP12, FcεRI-γ and CD3-ζ signaling subunits. For a comprehensive list of ITAM-signaling NK cell receptors, see Supplementary Table 1. Note that human CD16 can pair with homodimers of FcεRI-γ or CD3-ζ or with heterodimers of FcεRI-γ and CD3-ζ, whereas mouse CD16 signals efficiently only with homodimers of FcεRI-γ. ITAM-bearing signaling subunits contain aspartate residues (D) within their transmembrane segments that associate noncovalently with oppositely charged lysine or arginine residues within the transmembrane of the receptors, an exception being CD16, which also has an aspartate residue within its transmembrane. Y, tyrosine residues within ITAM domains.
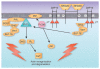
Figure 2
ITAM-mediated signaling in NK cells. ITAM-bearing signaling subunits are phosphorylated, probably by Src family kinases, after receptor engagement. Syk and/or ZAP-70 (both of which are expressed by human and mouse NK cells) are recruited to the phosphorylated ITAMs, initiating a cascade of downstream signaling as depicted. The signaling pathways depicted are hypothetical and were deduced by synthesizing results from many studies investigating ITAM-coupled receptor signaling in human and mouse NK cells. DAG, diacylglycerol; IP3, inositol-3,4,5-trisphosphate; PIP2, phosphatidylinositol-3,4-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate; pY, phosphotyrosine; ITK, tyrosine kinase; GADS and 3BP2, adaptor proteins; NFATp and NF-κB, transcription factors; PDK, phosphoinositide-dependent protein kinase; PKC-θ, protein kinase C-θ; RAF, mitogen-activated protein (MAP) kinase kinase kinase; RAS, GTPase.
Figure 3
DAP10-mediated signaling in NK cells. Cross-linking NKG2D causes NK cell activation that involves the recruitment of the p85 subunit of PI(3)K and recruitment of the Grb2-Vav1-Sos1 complex to the phosphorylated YINM motif in the cytoplasmic domain of DAP10. These events trigger distal signaling cascades as depicted.
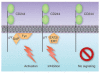
Figure 4
CD244 receptor complexes in NK cells. Phosphorylation of the tyrosines in the TIYXX(V/I) motifs in the cytoplasmic domain of CD244 can recruit the adaptor proteins SAP, EAT2 or ERT (ERT exists in mice, but not humans). SAP binds to Fyn to mediate signal transduction. It has been proposed that the CD244-SAP–Fyn complex is responsible for NK cell activation when NK cells encounter target cells expressing CD48, a ligand of CD244. Alternatively, evidence suggests that CD244-EAT2 and CD244-ERT complexes deliver inhibitory signals into NK cells, although this remains controversial,,,.
Similar articles
- NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway.
Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. Billadeau DD, et al. Nat Immunol. 2003 Jun;4(6):557-64. doi: 10.1038/ni929. Epub 2003 May 11. Nat Immunol. 2003. PMID: 12740575 - Natural killer cell signaling pathways.
Vivier E, Nunès JA, Vély F. Vivier E, et al. Science. 2004 Nov 26;306(5701):1517-9. doi: 10.1126/science.1103478. Science. 2004. PMID: 15567854 - Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways.
Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Coudert JD, et al. Blood. 2008 Apr 1;111(7):3571-8. doi: 10.1182/blood-2007-07-100057. Epub 2008 Jan 15. Blood. 2008. PMID: 18198346 - Physiologic functions of activating natural killer (NK) complex-encoded receptors on NK cells.
Ryan JC, Naper C, Hayashi S, Daws MR. Ryan JC, et al. Immunol Rev. 2001 Jun;181:126-37. doi: 10.1034/j.1600-065x.2001.1810110.x. Immunol Rev. 2001. PMID: 11513134 Review. - Activation of natural killer cells: underlying molecular mechanisms revealed.
Backström E, Kristensson K, Ljunggren HG. Backström E, et al. Scand J Immunol. 2004 Jul-Aug;60(1-2):14-22. doi: 10.1111/j.0300-9475.2004.01475.x. Scand J Immunol. 2004. PMID: 15238069 Review.
Cited by
- Rhomboid domain-containing protein 3 is a negative regulator of TLR3-triggered natural killer cell activation.
Liu J, Liu S, Xia M, Xu S, Wang C, Bao Y, Jiang M, Wu Y, Xu T, Cao X. Liu J, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7814-9. doi: 10.1073/pnas.1220466110. Epub 2013 Apr 22. Proc Natl Acad Sci U S A. 2013. PMID: 23610400 Free PMC article. - Immune and inflammatory role in renal disease.
Imig JD, Ryan MJ. Imig JD, et al. Compr Physiol. 2013 Apr;3(2):957-76. doi: 10.1002/cphy.c120028. Compr Physiol. 2013. PMID: 23720336 Free PMC article. Review. - The CD300 molecules: an emerging family of regulators of the immune system.
Borrego F. Borrego F. Blood. 2013 Mar 14;121(11):1951-60. doi: 10.1182/blood-2012-09-435057. Epub 2013 Jan 4. Blood. 2013. PMID: 23293083 Free PMC article. Review. - The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer.
Sedgwick AJ, Ghazanfari N, Constantinescu P, Mantamadiotis T, Barrow AD. Sedgwick AJ, et al. Front Immunol. 2020 Jul 31;11:1549. doi: 10.3389/fimmu.2020.01549. eCollection 2020. Front Immunol. 2020. PMID: 32903717 Free PMC article. Review.
References
- Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. - PubMed
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. - PubMed
- Tessmer MS, et al. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int. Immunol. 2007;19:391–400. - PubMed
- Alvarez-Arias DA, Campbell KS. Protein kinase C regulates expression and function of inhibitory killer cell Ig-like receptors in NK cells. J. Immunol. 2007;179:5281–5290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA105379/CA/NCI NIH HHS/United States
- P01 AI064520-040002/AI/NIAID NIH HHS/United States
- AI066897/AI/NIAID NIH HHS/United States
- CA095137/CA/NCI NIH HHS/United States
- AI64520/AI/NIAID NIH HHS/United States
- R01 CA095137-05/CA/NCI NIH HHS/United States
- R01 AI068129/AI/NIAID NIH HHS/United States
- R37 AI066897/AI/NIAID NIH HHS/United States
- R37 AI066897-08/AI/NIAID NIH HHS/United States
- R01 AI068129-08/AI/NIAID NIH HHS/United States
- CA105379/CA/NCI NIH HHS/United States
- R01 CA095137/CA/NCI NIH HHS/United States
- AI068129/AI/NIAID NIH HHS/United States
- P01 AI064520/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials