Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation - PubMed (original) (raw)
Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation
Clas B Johansson et al. Nat Cell Biol. 2008 May.
Abstract
Transplanted bone marrow-derived cells (BMDCs) have been reported to fuse with cells of diverse tissues, but the extremely low frequency of fusion has led to the view that such events are biologically insignificant. Nonetheless, in mice with a lethal recessive liver disease (tyrosinaemia), transplantation of wild-type BMDCs restored liver function by cell fusion and prevented death, indicating that cell fusion can have beneficial effects. Here we report that chronic inflammation resulting from severe dermatitis or autoimmune encephalitis leads to robust fusion of BMDCs with Purkinje neurons and formation of hundreds of binucleate heterokaryons per cerebellum, a 10-100-fold higher frequency than previously reported. Single haematopoietic stem-cell transplants showed that the fusogenic cell is from the haematopoietic lineage and parabiosis experiments revealed that fusion can occur without irradiation. Transplantation of rat bone marrow into mice led to activation of dormant rat Purkinje neuron-specific genes in BMDC nuclei after fusion with mouse Purkinje neurons, consistent with nuclear reprogramming. The precise neurological role of these heterokaryons awaits elucidation, but their frequency in brain after inflammation is clearly much higher than previously appreciated.
Figures
Figure 1
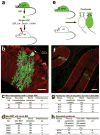
Progeny of a single HSC can form heterokaryons with Purkinje neurons and the formation of cerebellar heterokaryons is independent of variables associated with bone marrow transplantation. (a) Schematic of the experimental design for BMT. Bone marrow from GFP mice was harvested and enriched for HSCs by FACS. A single HSC was transplanted with 106 helper cells into a lethally irradiated wild type mouse. (b) A high power laser-scanning confocal image from a cerebellar sagittal section of a mouse transplanted with a single HSC. Immunohistochemistry with specific antibodies demonstrates the presence of GFP in a single cerebellar heterokaryon (green/yellow) co-expressing calbindin (red/yellow) in Purkinje neurons. The GFP+ Purkinje neuron is located in the Purkinje cell layer (PCL) with dendrites extending into the molecular layer (ML). The only output connection from the cerebellum to the cerebellar nucleus is mediated by a single axon (arrow) descending through the granular cell layer (GCL). (c, d) Quantification of heterokaryon formation in the single HSC and whole BM transplantation paradigm, respectively. (e) Schematic of the experimental design to form parabionts. Age- and weight-matched female mice (a wild type, recipient and a transgenic, ubiquitously expressing GFP, donor) are surgically joined from the olecranon to the knee. (f) Immunohistochemistry demonstrates the presence of two heterokaryons, (GFP+ Purkinje neurons), the fusion product of a GFP+ BMDC and a Purkinje neuron expressing calbindin (yellow/red). (g) Quantification of cerebellar heterokaryons formed in conjoined parabionts without ulcerative dermatitis for 20 or 26 weeks. No gross pathology was detected in these mice such as dermatitis. (h) Quantification of cerebellar heterokaryons following separation of parabionts. Parabionts were conjoined for 38 weeks before being separated for 20 weeks, during which blood chimerism fell from 51 and 56% to 6 and 10%, respectively. Although de novo heterokaryon production cannot be ruled out since some GFP+ cells remained present in the circulation, the presence of heterokaryons when GFP+ cells were markedly reduced in the circulation is consistent with their persistence over time. Scale bars (a) 50 μm and (b) 100 μm.
Figure 2
Idiopathic ulcerative dermatitis modulates cerebellar heterokaryons. A subset of parabionts developed a chronic dermatitis, consistent with idiopathic ulcerative dermatitis. (a, b) Hematoxylin and eosin (H&E) staining of skin biopsies from a parabiont without dermatitis (a) and a parabiont with severe dermatitis (b). (a) Normal skin with all dermal structures, a continuous two-three-cell layer, epidermis (ED), dermis (DE), subcutaneous fat, and panniculus carnosus (PC). (b) Severe dermatitis. The skin is characterized by ulceration, serocellular crusting (SC) total loss of the epidermis and necrosis of the superficial dermis. Normal dermal structures are absent and the dermis is severely thickened by fibrosis and chronic inflammation extending to the panniculus carnosus (PC). (c, d) Representative FACS plots of PB from a normal mouse (c) and from a mouse with dermatitis (d) demonstrating the presence of inflammatory cells in normal mice and in mice with dermatitis. The peripheral blood was stained for CD45PE, CD11b-biotin-APC and Gr-1-biotin-APC and analyzed with a FACSCalibur using Flow-Jo and CD45+ cells were plotted as CD11b/Gr-1+ and GFP+. (e, f) Cerebellar sagittal sections from a mouse (#21) with severe dermatitis. Immunohistochemistry with antibodies, to GFP (green) and to calbindin (red) demonstrates that numerous heterokaryons have been formed in the cerebellum. (f) A high-power laser-scanning confocal image of heterokaryons shows their normal morphology with GFP+ cell bodies, dendrites extending into the molecular layer and an axon descending through the granular cell layer. Scale bars (a, b) 200 μm, (e) 100 μm, and (f) 20 μm.
Figure 3
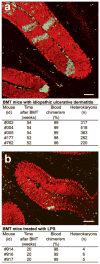
Heterokaryon formation in bone marrow transplanted mice with inflammation due to idiopathic ulcerative dermatitis increased but not in LPS induced inflammation. (a, b) Immunohistochemistry of cerebellar sagittal sections with antibodies to GFP (green) and to calbindin (red) show several green/yellow heterokaryons. (a) Several hundred heterokaryons were detected in cerebella of BMT mice 52–54 weeks post transplantation. (b) Few heterokaryons were formed in the cerebella of mice treated for four weeks with intraperitoneal injections of LPS. Note the increased infiltration of GFP+ macrophages/microglia cells in BMT and the LPS treated mice relative to parabionts in (Fig. 1f, 2e). Scale bar 100 μm.
Figure 4
Increased heterokaryon formation in mice with experimental autoimmune encephalitis (EAE). (a–d) Immunohistochemistry of cerebellar sagittal sections with antibodies to GFP (green) and to calbindin (red). (a) Low power laser-scanning confocal image shows extensive infiltration of GFP+ macrophages/microglia cells and a heterokaryon with a GFP+ cell body (arrow) in sagittal section from mouse #0121. (b) High power laser-scanning image shows a heterokaryon (arrow in a) with dendrites in the molecular layer and a descending axon. Several macrophages/microglia cells are present around the heterokaryon and in the granular cell layer. (c) Low power laser-scanning image from an area with a major loss of Purkinje neurons in a sagittal section from mouse #0127 in one region with no GFP+ cell bodies (arrows) but not in a neighboring region with a number of GFP+ cell bodies (box). (d) High power confocal image of the boxed area in (c) shows several heterokaryons with complex dendrites and many synaptic spines intermingled with macrophages/microglia cells despite the intense inflammation. Scale bar in (a, c) 100 μm, and (b, d) 20 μm.
Figure 5
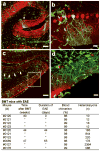
Rat neural gene expression in heterokaryons of mice transplanted with rat bone marrow. (a) Representative FACS analysis of PB of a mouse after BMT with rat BM ubiquitously expressing GFP. More than 93% of the PB is derived from the rat BM. (b) Immunohistochemistry with antibodies to GFP (green) and calbindin (red) demonstrate rat-mouse chimeric heterokaryons and rat macrophage/microglia cells in a sagittal section from the cerebellum (mouse #933). (c) Quantification of cerebellar heterokaryons in mice BMT with rat BM. (d) RT-PCR products were resolved on an agarose gel. RT-PCR products show the expression of the rat Purkinje neuron genes Calb1, L7/pcp-2, and Gsbs in addition to Kcnc1 from mice transplanted with rat BM. Primers for rat and mouse Gapdh were used as internal controls. Scale bar 100 μm.
Comment in
- Inflammation as a matchmaker: revisiting cell fusion.
Singec I, Snyder EY. Singec I, et al. Nat Cell Biol. 2008 May;10(5):503-5. doi: 10.1038/ncb0508-503. Nat Cell Biol. 2008. PMID: 18454127 No abstract available.
Similar articles
- Aberrant cerebellar Purkinje cell function repaired in vivo by fusion with infiltrating bone marrow-derived cells.
Kemp KC, Dey R, Verhagen J, Scolding NJ, Usowicz MM, Wilkins A. Kemp KC, et al. Acta Neuropathol. 2018 Jun;135(6):907-921. doi: 10.1007/s00401-018-1833-z. Epub 2018 Mar 14. Acta Neuropathol. 2018. PMID: 29541917 Free PMC article. - Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion.
Nygren JM, Liuba K, Breitbach M, Stott S, Thorén L, Roell W, Geisen C, Sasse P, Kirik D, Björklund A, Nerlov C, Fleischmann BK, Jovinge S, Jacobsen SE. Nygren JM, et al. Nat Cell Biol. 2008 May;10(5):584-92. doi: 10.1038/ncb1721. Epub 2008 Apr 20. Nat Cell Biol. 2008. PMID: 18425115 - Radiation damage increases Purkinje neuron heterokaryons in neonatal cerebellum.
Espejel S, Romero R, Alvarez-Buylla A. Espejel S, et al. Ann Neurol. 2009 Jul;66(1):100-9. doi: 10.1002/ana.21670. Ann Neurol. 2009. PMID: 19670439 Free PMC article. - Cell fusion and reprogramming: resolving our transdifferences.
Rodić N, Rutenberg MS, Terada N. Rodić N, et al. Trends Mol Med. 2004 Mar;10(3):93-6. doi: 10.1016/j.molmed.2004.01.010. Trends Mol Med. 2004. PMID: 15106589 Review. - Bone marrow-derived cells: A potential approach for the treatment of xerostomia.
Tran SD, Sumita Y, Khalili S. Tran SD, et al. Int J Biochem Cell Biol. 2011 Jan;43(1):5-9. doi: 10.1016/j.biocel.2010.10.010. Epub 2010 Oct 28. Int J Biochem Cell Biol. 2011. PMID: 21035563 Review.
Cited by
- Characterization of cell fusion in an experimental mouse model of endometriosis†.
Tal A, Tal R, Shaikh S, Gidicsin S, Mamillapalli R, Taylor HS. Tal A, et al. Biol Reprod. 2019 Feb 1;100(2):390-397. doi: 10.1093/biolre/ioy221. Biol Reprod. 2019. PMID: 30304517 Free PMC article. - Intrinsic signalling factors associated with cancer cell-cell fusion.
Dittmar T, Hass R. Dittmar T, et al. Cell Commun Signal. 2023 Apr 4;21(1):68. doi: 10.1186/s12964-023-01085-5. Cell Commun Signal. 2023. PMID: 37016404 Free PMC article. Review. - Development of Donor Recipient Chimeric Cells of bone marrow origin as a novel approach for tolerance induction in transplantation.
Cwykiel J, Madajka-Niemeyer M, Siemionow M. Cwykiel J, et al. Stem Cell Investig. 2021 Apr 19;8:8. doi: 10.21037/sci-2020-044. eCollection 2021. Stem Cell Investig. 2021. PMID: 33969113 Free PMC article. - Karyotype Aberrations in Action: The Evolution of Cancer Genomes and the Tumor Microenvironment.
Baudoin NC, Bloomfield M. Baudoin NC, et al. Genes (Basel). 2021 Apr 12;12(4):558. doi: 10.3390/genes12040558. Genes (Basel). 2021. PMID: 33921421 Free PMC article. Review. - The Hallmarks of Circulating Hybrid Cells.
Patel RK, Parappilly M, Rahman S, Schwantes IR, Sewell M, Giske NR, Whalen RM, Durmus NG, Wong MH. Patel RK, et al. Results Probl Cell Differ. 2024;71:467-485. doi: 10.1007/978-3-031-37936-9_21. Results Probl Cell Differ. 2024. PMID: 37996690 Review.
References
- Ferrari G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. - PubMed
- Bittner RE, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anatomy and embryology. 1999;199:391–396. - PubMed
- Lagasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. - PubMed
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. - PubMed
- Fukada S, et al. Muscle regeneration by reconstitution with bone marrow or fetal liver cells from green fluorescent protein-gene transgenic mice. Journal of cell science. 2002;115:1285–1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG009521/AG/NIA NIH HHS/United States
- AG024987/AG/NIA NIH HHS/United States
- AG020961/AG/NIA NIH HHS/United States
- R01 AG024987/AG/NIA NIH HHS/United States
- AG009521/AG/NIA NIH HHS/United States
- R01 AG020961/AG/NIA NIH HHS/United States
- R01 AG009521/AG/NIA NIH HHS/United States
- R01 HD018179/HD/NICHD NIH HHS/United States
- HD018179/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous