Roles of calcium-stimulated adenylyl cyclase and calmodulin-dependent protein kinase IV in the regulation of FMRP by group I metabotropic glutamate receptors - PubMed (original) (raw)
Comparative Study
Roles of calcium-stimulated adenylyl cyclase and calmodulin-dependent protein kinase IV in the regulation of FMRP by group I metabotropic glutamate receptors
Hansen Wang et al. J Neurosci. 2008.
Abstract
The fragile X syndrome is caused by the lack of fragile X mental retardation protein (FMRP) attributable to silencing of the FMR1 gene. The metabotropic glutamate receptors (mGluRs) in the CNS contribute to different brain functions, including learning/memory, mental disorders, drug addiction, and persistent pain. Most of the previous studies have been focused on downstream targets of FMRP in hippocampal neurons, and fewer studies have been reported for the second-messenger signaling pathways between group I mGluRs and FMRP. Furthermore, no molecular study has been performed in the anterior cingulate cortex (ACC), a key region involved in high brain cognitive and executive functions. In this study, we demonstrate that activation of group I mGluR upregulated FMRP in ACC neurons of adult mice through the Ca(2+)-dependent signaling pathways. Using genetic approaches, we found that Ca(2+)/calmodulin-stimulated adenylyl cyclase 1 (AC1) and calcium/calmodulin-dependent kinase IV (CaMKIV) contribute to the upregulation of FMRP induced by stimulating group I mGluRs. The upregulation of FMRP occurs at the transcriptional level. The cAMP-dependent protein kinase is activated by stimulating group I mGluRs through AC1 in ACC neurons. Both AC1 and CaMKIV contribute to the regulation of FMRP by group I mGluRs probably through cAMP response element-binding protein activation. Our study has provided the first evidence for a molecular link between group I mGluRs and FMRP in ACC neurons and may help us to understand the pathogenesis of fragile X syndrome.
Figures
Figure 1.
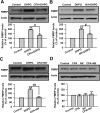
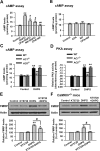
Activation of group I mGluRs upregulates FMRP in ACC neurons. A, Immunohistochemistry of FMRP in ACC. Compared with the control (left), the increase of the immunoreactivity of FMRP by DHPG (100 μ
m
, 30 min; right) could be found in neurons from layers II–VI in ACC. A high-magnification image of the selected part showing the staining in layers II and III is provided at the bottom. Scale bars: top, 100 μm; bottom, 25 μm. B, Application of the group I mGluR agonist DHPG (100 μ
m
) for 30 min increased the levels of FMRP in ACC, as measured by Western blot. C, Application of the group II mGluR agonist (2_R_,4_R_)-APDC (10 μ
m
) or group III mGluR agonist ACPT-I (100 μ
m
) for 30 min did not affect the levels of FMRP in ACC slices. D, The levels of FMRP were increased by DHPG (10–150 μ
m
) in a dose-dependent manner. The highest level of FMRP was seen at 100 μ
m
; DHPG at higher concentration (150 μ
m
) did not cause additional increase of FMRP. E, DHPG (100 μ
m
) increased FMRP in a time-dependent manner, the increase was observed at 15 min, and the highest increase was reached at 30 min. F, The increase of FMRP caused by DHPG was partially blocked by the selective mGluR1 antagonist MPMQ (10 μ
m
) or a specific mGluR5 antagonist of ACDPP (10 μ
m
); the presence of the group I mGluR antagonist
dl
-AP-3 (100 μ
m
) completely blocked the increase of FMRP caused by DHPG in ACC slices. The antagonists were applied to slices 20 min before and during the DHPG treatment. Representative Western blot (top) and quantification data (bottom) of FMRP levels are shown for corresponding treatments from B–F. Data were normalized by the control values. *p < 0.05, **p < 0.01 compared with control; #p < 0.05, ##p < 0.01 compared with DHPG treatment; n = 3 mice for each group in A; n = 6 mice for each group in B; n = 4 mice for each group in C–F.
Figure 2.
Calcium mediates the regulation of FMRP by group I mGluRs in ACC neurons. A, The SERCA pump inhibitor CPA (50 μ
m
) partially blocked the upregulation of FMRP by DHPG (100 μ
m
, 30 min). Cyclopiazonic acid was applied to slices 20 min before and during DHPG treatment. B, L-type Ca2+ channels blocker Nif (25 μ
m
) partially blocked the increase of FMRP caused by DHPG treatment. Nifedipine was applied to slices 20 min before and during DHPG treatment. C, Coapplication of cyclopiazonic acid with nifedipine almost completely blocked the upregulation of FMRP by DHPG treatment. CPA (50 μ
m
) and Nif (25 μ
m
) were applied to slices 20 min before and during DHPG treatment. D, Application of cyclopiazonic acid (50 μ
m
), nifedipine (25 μ
m
), or coapplication of cyclopiazonic acid with nifedipine for 30 min did not affect the basal levels of FMRP in ACC slices. Representative Western blot (top) and quantification data (bottom) of FMRP are shown for the corresponding treatments. Data were normalized by the control values. *p < 0.05, **p < 0.01 compared with control; #p < 0.05, ##p < 0.01 compared with DHPG treatment; n = 6 mice for each group in A–C; n = 4 mice for each group in D.
Figure 3.
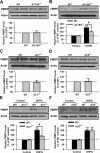
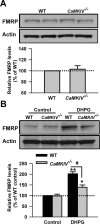
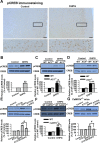
Upregulation of FMRP was partially blocked in ACC from mice lacking AC1. A, There was no difference in the basal levels of FMRP in ACC slices between WT and AC1&8 DKO mice, as shown by Western blot. B, The increase of FMRP in ACC slices treated by DHPG (100 μ
m
, 30 min) was partially blocked in AC1&8 DKO mice compared with WT mice. C, D, There was no difference in the basal levels of FMRP in ACC slices between WT and AC1 KO (C) or AC8 KO (D) mice, as shown by Western blot. E, The increase of FMRP in ACC slices from AC1 KO mice was attenuated compared with WT mice. The slices were treated with DHPG (100 μ
m
) for 30 min. F, The increase of FMRP attributable to DHPG treatment (100 μ
m
, 30 min) in ACC slices from AC8 KO mice was not different from that in WT mice. Representative Western blot (top) and quantification data (bottom) of FMRP are shown for the corresponding treatments. Data were normalized by the WT control values. *p < 0.05, **p < 0.01 compared with control; #p < 0.05 compared with WT; n = 4 mice for each group in A and B; n = 6 mice for each group in C–F.
Figure 4.
CaMKIV contributes to the upregulation of FMRP by group I mGluRs in ACC neurons. A, There was no difference in the basal levels of FMRP in ACC slices between WT and CaMKIV KO mice. B, The increase of FMRP after treatment with DHPG (100 μ
m
) for 30 min was attenuated in ACC slices from CaMKIV KO mice compared with WT mice. Representative Western blot (top) and quantification data (bottom) of FMRP are shown for the corresponding treatments. Data were normalized by WT control values. *p < 0.05, **p < 0.01 compared with control; #p < 0.05 compared with WT; n = 4 mice for each group.
Figure 5.
The upregulation of FMRP by group I mGluRs occurs at the transcriptional level. A, DNA transcription inhibitor actinomycin D (40 μ
m
) abolished the DHPG-induced increase of FMRP in ACC slices, as shown by Western blot. Actinomycin D was applied to slices 20 min before and during DHPG (100 μ
m
) treatment for 30 min. B, DHPG (100 μ
m
, 15 min) treatment increased the levels of Fmr1 mRNA in ACC slices, as shown by RT-PCR. C, D, The increase of Fmr1 mRNA was attenuated in ACC slices from AC1 KO (C) or CaMKIV KO (D) mice compared with WT mice, as shown by RT-PCR. The size of PCR products is 141 and 191 bp for Fmr1 and GAPDH, respectively. Representative gels (top) and quantification data (bottom) of FMRP or Fmr1 mRNA are shown for the corresponding treatments. Data were normalized by WT control values. *p < 0.05, **p < 0.01 compared with control; #p < 0.05 compared with WT; n = 4 mice for each group.
Figure 6.
PKA is activated by stimulating group I mGluRs in ACC neurons. A, The cAMP levels was increased in ACC slices by DHPG (100 μ
m
, 15 min) treatment as shown by cAMP assay. Both CPA (50 μ
m
) and Nif (25 μ
m
) partially blocked the increase of cAMP caused by DHPG. Coapplication of cyclopiazonic acid with nifedipine completely blocked the increase of cAMP caused by DHPG treatment. CPA (50 μ
m
) or Nif (25 μ
m
) was applied to slices 20 min before and during DHPG treatment. B, Application of cyclopiazonic acid (50 μ
m
), nifedipine (25 μ
m
), or coapplication of cyclopiazonic acid with nifedipine for 30 min did not affect the basal levels of cAMP in ACC slices. C, The increase of cAMP was abolished in AC1 KO but not in AC8 KO mice. D, The PKA activity was increased in ACC slices by DHPG (100 μ
m
, 15 min) treatment, as shown by active PKA assay. The increase of the PKA activity was abolished in AC1 KO but not in AC8 KO mice. E, The PKA inhibitor KT5720 (10 μ
m
) partially blocked the increase of FMRP caused by DHPG treatment. KT5720 was applied to slices 15 min before and during DHPG treatment. KT5720 (10 μ
m
, 30 min) did affect the basal levels of FMRP in the ACC neurons. F, KT5720 (10 μ
m
) abolished the increase of FMRP caused by DHPG treatment in ACC slices from CaMKIV KO mice. KT5720 was applied to slices 15 min before and during DHPG treatment. Representative Western blot (top) and quantification data (bottom) of FMRP are shown for the corresponding treatment in E and F. Data were normalized by the control values in E and F. *p < 0.05, **p < 0.01 compared with control in A–F; #p < 0.05, ##p < 0.01 compared with DHPG treatment in A, E, and F; ##p < 0.01 compared with WT in C and D; n = 4 mice for each group.
Figure 7.
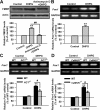
AC1 and CaMKIV contribute to the phosphorylation of CREB by group I mGluR activation. A, Immunohistochemistry of pCREB in ACC. Compared with the control (left), the increase of the immunoreactivity of pCREB caused by DHPG (100 μ
m
, 15 min; right) could be found in neurons from layers II–VI in ACC. A high-magnification image of the selected region showing the staining in layers II–III is provided at the bottom. Scale bars: top, 100 μm; bottom, 20 μm. B, Activation of group I mGluRs by DHPG induced the phosphorylation of CREB in ACC slices. ACC slices were treated by DHPG (100 μ
m
) for 15 min, and the phosphorylation of CREB at Ser133 residue was tested by Western blot. C, The phosphorylation of CREB induced by DHPG (100 μ
m
, 15 min) treatment was significantly attenuated in ACC slices from AC1 KO mice compared with WT mice. The basal phosphorylation levels of CREB were not changed in ACC slices from AC1 KO mice. D, There is no difference in the phosphorylation levels of CREB after DHPG (100 μ
m
, 15 min) treatment in ACC slices between AC8 KO and WT mice. The basal phosphorylation levels of CREB were not changed in ACC slices from AC8 KO mice. E, The PKA inhibitor KT5720 (10 μ
m
) partially blocked the increase of pCREB caused by DHPG treatment. KT5720 was applied to slices 15 min before and during DHPG treatment. KT5720 (10 μ
m
, 15 min) did affect the basal levels of pCREB in ACC neurons. F, The phosphorylation of CREB induced by DHPG (100 μ
m
, 15 min) treatment was significantly attenuated in ACC slices from CaMKIV KO mice compared with WT mice. The basal phosphorylation levels of CREB were not changed in ACC slices from CaMKIV KO mice. G, KT5720 (10 μ
m
) abolished the increase of pCREB caused by DHPG treatment in ACC slices from CaMKIV KO mice. KT5720 was applied to slices 15 min before and during DHPG treatment. Representative Western blot (top) and quantification data (bottom) of pCREB levels are shown for corresponding treatments. Data were normalized by the control values in B, E, and G and by the WT control values in C, D, and F; *p < 0.05, **p < 0.01 compared with control from B–G; #p < 0.05 compared with WT in C and F; #p < 0.05 compared with DHPG treatment in E and G; n = 3 mice for each group in experiments in A; n = 6 mice for each group in experiments in B; n = 4 mice for each group in experiments from C–G.
Figure 8.
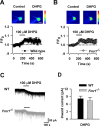
The function of group I mGluRs in ACC neurons is not affected in Fmr1 KO mice. A, Represent images (top) and time plot (bottom) showing that DHPG (100 μ
m
) perfusion for 2 min increased the Ca2+ signals in ACC neurons from WT mice (n = 6 cells). Scale bar, 20 μm. B, Similar increase of Ca2+ signals were also found in ACC neurons from Fmr1 KO mice (n = 8 cells); p > 0.05 compared with that in A. C, Typical traces showing the inward current induced by perfusion of DHPG (100 μ
m
) for 2 min. D, Pooled data showing that there is no difference in DHPG-induced currents between WT (n = 11 cells) and _Fmr1_−/− (n = 8 cells) mice; p > 0.05 compared with WT.
Figure 9.
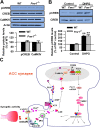
FMRP is downstream of the group I mGluR–CREB pathway in ACC neurons. A, There is no difference in basal levels of CREB in ACC between WT and Fmr1 KO mice. The basal levels of CaMKIV were not affected in ACC of Fmr1 KO mice compared with that of WT mice. B, The phosphorylation of CREB induced by DHPG treatment was not affected in Fmr1 KO mice compared with WT mice. The slices were treated with DHPG (100 μ
m
) for 15 min. Representative Western blot (top) and quantification data (bottom) of pCREB levels are shown for corresponding treatments. Data were normalized by WT control values. **p < 0.01 compared with control; n = 4 mice for each group in A; n = 6 mice in B. C, The proposed signaling pathway for the regulation of FMRP by group I mGluRs in ACC neurons. Activation of group I mGluR triggers the Ca2+ release from intracellular calcium stores by IP3 and Ca2+ influx from L-VDCCs through membrane depolarization. Postsynaptic increases in Ca2+ leads to activation of Ca2+–calmodulin (CaM)-dependent pathways. Among them, Ca2+ and CaM-stimulated AC1 is activated, and this activation leads to the generation of the key second-messenger cAMP. Subsequently, cAMP activates the PKA. PKA then translocates to the nucleus and phosphorylates CREB. In addition to the cAMP–PKA pathway, rapid CaM translocation into the nucleus activates CaMKIV. CaMKIV then phosphorylates CREB. pCREB initiates the CREB-dependent transcription of Fmr1 gene and upregulates FMRP in the cytoplasm. FMRP may interact with several FRMP interactors and causes changes in neuronal functions in ACC.
Similar articles
- Ca2+/calmodulin-dependent protein kinase IV links group I metabotropic glutamate receptors to fragile X mental retardation protein in cingulate cortex.
Wang H, Fukushima H, Kida S, Zhuo M. Wang H, et al. J Biol Chem. 2009 Jul 10;284(28):18953-62. doi: 10.1074/jbc.M109.019141. Epub 2009 May 12. J Biol Chem. 2009. PMID: 19436069 Free PMC article. - Roles of CREB in the regulation of FMRP by group I metabotropic glutamate receptors in cingulate cortex.
Wang H, Morishita Y, Miura D, Naranjo JR, Kida S, Zhuo M. Wang H, et al. Mol Brain. 2012 Aug 6;5:27. doi: 10.1186/1756-6606-5-27. Mol Brain. 2012. PMID: 22867433 Free PMC article. - Dual regulation of fragile X mental retardation protein by group I metabotropic glutamate receptors controls translation-dependent epileptogenesis in the hippocampus.
Zhao W, Chuang SC, Bianchi R, Wong RK. Zhao W, et al. J Neurosci. 2011 Jan 12;31(2):725-34. doi: 10.1523/JNEUROSCI.2915-10.2011. J Neurosci. 2011. PMID: 21228181 Free PMC article. - Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse.
Ronesi JA, Huber KM. Ronesi JA, et al. Sci Signal. 2008 Feb 5;1(5):pe6. doi: 10.1126/stke.15pe6. Sci Signal. 2008. PMID: 18272470 Review. - The pathophysiology of fragile X (and what it teaches us about synapses).
Bhakar AL, Dölen G, Bear MF. Bhakar AL, et al. Annu Rev Neurosci. 2012;35:417-43. doi: 10.1146/annurev-neuro-060909-153138. Epub 2012 Apr 5. Annu Rev Neurosci. 2012. PMID: 22483044 Free PMC article. Review.
Cited by
- Activity-dependent FMRP requirements in development of the neural circuitry of learning and memory.
Doll CA, Broadie K. Doll CA, et al. Development. 2015 Apr 1;142(7):1346-56. doi: 10.1242/dev.117127. Development. 2015. PMID: 25804740 Free PMC article. - Analgesic effects of adenylyl cyclase inhibitor NB001 on bone cancer pain in a mouse model.
Kang WB, Yang Q, Guo YY, Wang L, Wang DS, Cheng Q, Li XM, Tang J, Zhao JN, Liu G, Zhuo M, Zhao MG. Kang WB, et al. Mol Pain. 2016 Sep 9;12:1744806916652409. doi: 10.1177/1744806916652409. Print 2016. Mol Pain. 2016. PMID: 27612915 Free PMC article. - Common variants in genes of the postsynaptic FMRP signalling pathway are risk factors for autism spectrum disorders.
Waltes R, Duketis E, Knapp M, Anney RJ, Huguet G, Schlitt S, Jarczok TA, Sachse M, Kämpfer LM, Kleinböck T, Poustka F, Bölte S, Schmötzer G, Voran A, Huy E, Meyer J, Bourgeron T, Klauck SM, Freitag CM, Chiocchetti AG. Waltes R, et al. Hum Genet. 2014 Jun;133(6):781-92. doi: 10.1007/s00439-013-1416-y. Epub 2014 Jan 19. Hum Genet. 2014. PMID: 24442360 - Activity-dependent modulation of neural circuit synaptic connectivity.
Tessier CR, Broadie K. Tessier CR, et al. Front Mol Neurosci. 2009 Jul 30;2:8. doi: 10.3389/neuro.02.008.2009. eCollection 2009. Front Mol Neurosci. 2009. PMID: 19668708 Free PMC article. - Postsynaptic activity reverses the sign of the acetylcholine-induced long-term plasticity of GABAA inhibition.
Domínguez S, Fernández de Sevilla D, Buño W. Domínguez S, et al. Proc Natl Acad Sci U S A. 2014 Jul 1;111(26):E2741-50. doi: 10.1073/pnas.1321777111. Epub 2014 Jun 17. Proc Natl Acad Sci U S A. 2014. PMID: 24938789 Free PMC article.
References
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221–1225. - PubMed
- Bianchi R, Young SR, Wong RK. Group I mGluR activation causes voltage-dependent and -independent Ca2+ rises in hippocampal pyramidal cells. J Neurophysiol. 1999;81:2903–2913. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous