Coordination of early protective immunity to viral infection by regulatory T cells - PubMed (original) (raw)
Coordination of early protective immunity to viral infection by regulatory T cells
Jennifer M Lund et al. Science. 2008.
Abstract
Suppression of immune responses by regulatory T cells (Tregs) is thought to limit late stages of pathogen-specific immunity as a means of minimizing associated tissue damage. We examined a role for Tregs during mucosal herpes simplex virus infection in mice, and observed an accelerated fatal infection with increased viral loads in the mucosa and central nervous system after ablation of Tregs. Although augmented interferon production was detected in the draining lymph nodes (dLNs) in Treg-deprived mice, it was profoundly reduced at the infection site. This was associated with a delay in the arrival of natural killer cells, dendritic cells, and T cells to the site of infection and a sharp increase in proinflammatory chemokine levels in the dLNs. Our results suggest that Tregs facilitate early protective responses to local viral infection by allowing a timely entry of immune cells into infected tissue.
Figures
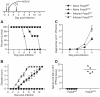
Fig. 1
Tregs respond to local HSV-2 infection with kinetics similar to those of effector CD4+ T cells. (A to F) Foxp3gfp mice were infected with HSV-2, and vaginas and dLNs were prepared for analysis by flow cytometry. CD4+ Tcells are CD4+Foxp3- and Tregs are CD4+Foxp3+. CD4+ Tcells (C) and Tregs (D) in the dLNs were stained for inducible costimulatory molecule expression. Mice were administered BrdU in the drinking water throughout the course of infection (E and F), and BrdU incorporation by CD4+ T cells and Tregs was detected by flow cytometry. (G) Effector T cells and antigen-pulsed APCs were cocultured with Foxp3+ suppressor cells as indicated, 3H was added, and cells were cultured for an additional 20 hours before harvest and measurement of proliferation with a beta-counter.
Fig. 2
Tregs are required to prevent early death from HSV-2 infection. Foxp3WT or Foxp3DTR mice were infected with HSV-2 and treated with DT. Survival (A) and diseasescore (B) were monitored daily and vaginal washes were collected daily to assess vaginal viral titer (C) by plaque assay on Vero cells. (D) Spinal cords were collected 4 days after infection and plaque assays were performed on homogenates.
Fig. 3
Tregs are required to mount a protective immune response at the site of infection subsequent to genital HSV-2 infection. Foxp3WT or Foxp3DTR mice were infected with HSV-2 and treated with DT. (A) For ex vivo cultures, CD4+ T cells were isolated from the dLNs of naïve or infected Foxp3WT or Foxp3DTR mice and cultured for 3 days with irradiated, heat-inactivated HSV-2-pulsed APCs. IFN-γ was detected by enzyme-linked immunosorbent assay (ELISA). (B) Mice were infected and treated with DT as in Fig. 2, dLNs were collected 2 days after infection, and extracts were prepared for IFN-α detection by ELISA. (C and D) IFN-γ present in vaginal washes collected at various times after infection (C) and IFN-α from vaginal washes collected 2 days after infection (D) were measured by ELISA. (E) At the indicated times after infection, dLNs and vaginal tracts were subjected to flow cytometric analysis. Naïve mice received DT according to the same schedule as infected mice.
Fig. 4
Tregs modulate the chemokine gradient that controls proper effector cell homing to the dLNs and site of infection. (A) NK cells, CD11b+ DCs, and pDCs from naïve donors or CD4+ T cells isolated from the dLNs of Foxp3gfp donor mice infected with HSV-2 for 6 days were labeled with CFSE and injected into recipient mice. Recipients were Foxp3WT or Foxp3DTR mice treated with DT and infected with HSV-2, and mice received CFSE-labeled cells 2 days after infection. Twenty-four hours after transfer, dLNs and vaginal tracts from recipient mice were examined for percentage of the indicated population that was CFSE+ by flow cytometry. (B) Foxp3WT or Foxp3DTR mice were infected with HSV-2 and treated with DT. Two days after infection, dLNs and vaginal tract extracts were prepared for chemokine detection by Luminex bead assay. (C) Chemokine mRNA amounts in DCs and CD45- stromal cells were measured by real-time PCR and are shown as the mean of three independent experiments relative to hypoxanthine-guanine phosphoribosyl transferase expression. (D) Foxp3_DTR_ mice were treated with DT and infected with LCMV. Five days after infection, livers were collected and assayed for viral titer, and LNs were analyzed for chemokines as in (B).
Comment in
- Immunology. Immunity benefits from a little suppression.
Kassiotis G, O'Garra A. Kassiotis G, et al. Science. 2008 May 30;320(5880):1168-9. doi: 10.1126/science.1159090. Science. 2008. PMID: 18511677 No abstract available.
Similar articles
- Regulatory T cells are essential to promote proper CD4 T-cell priming upon mucosal infection.
Soerens AG, Da Costa A, Lund JM. Soerens AG, et al. Mucosal Immunol. 2016 Nov;9(6):1395-1406. doi: 10.1038/mi.2016.19. Epub 2016 Mar 23. Mucosal Immunol. 2016. PMID: 27007674 Free PMC article. - Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2.
Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Zhao X, et al. J Exp Med. 2003 Jan 20;197(2):153-62. doi: 10.1084/jem.20021109. J Exp Med. 2003. PMID: 12538655 Free PMC article. - CXCR3 deficiency increases susceptibility to genital herpes simplex virus type 2 infection: Uncoupling of CD8+ T-cell effector function but not migration.
Thapa M, Carr DJ. Thapa M, et al. J Virol. 2009 Sep;83(18):9486-501. doi: 10.1128/JVI.00854-09. Epub 2009 Jul 8. J Virol. 2009. PMID: 19587047 Free PMC article. - Innate immunity to herpes simplex virus type 2.
Duerst RJ, Morrison LA. Duerst RJ, et al. Viral Immunol. 2003;16(4):475-90. doi: 10.1089/088282403771926300. Viral Immunol. 2003. PMID: 14733735 Review. - Vaginal immunity in the HSV-2 mouse model.
Parr MB, Parr EL. Parr MB, et al. Int Rev Immunol. 2003 Jan-Feb;22(1):43-63. doi: 10.1080/08830180305228. Int Rev Immunol. 2003. PMID: 12710503 Review.
Cited by
- The role of CD4+FoxP3+ regulatory T cells in the immunopathogenesis of COVID-19: implications for treatment.
Wang Y, Zheng J, Islam MS, Yang Y, Hu Y, Chen X. Wang Y, et al. Int J Biol Sci. 2021 Apr 10;17(6):1507-1520. doi: 10.7150/ijbs.59534. eCollection 2021. Int J Biol Sci. 2021. PMID: 33907514 Free PMC article. Review. - Regulatory T cells in infection.
Maizels RM, Smith KA. Maizels RM, et al. Adv Immunol. 2011;112:73-136. doi: 10.1016/B978-0-12-387827-4.00003-6. Adv Immunol. 2011. PMID: 22118407 Free PMC article. Review. - Use of transcriptional profiling to delineate the initial response of mice to intravaginal herpes simplex virus type 2 infection.
Cherpes TL, Harvey SA, Phillips JM, Vicetti Miguel RD, Melan MA, Quispe Calla NE, Hendricks RL. Cherpes TL, et al. Viral Immunol. 2013 Jun;26(3):172-9. doi: 10.1089/vim.2012.0093. Epub 2013 May 2. Viral Immunol. 2013. PMID: 23638732 Free PMC article. - Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model.
Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS, Martuza RL, Rabkin SD. Cheema TA, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):12006-11. doi: 10.1073/pnas.1307935110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754388 Free PMC article. - Herpes simplex virus-2 glycoprotein interaction with HVEM influences virus-specific recall cellular responses at the mucosa.
Kopp SJ, Storti CS, Muller WJ. Kopp SJ, et al. Clin Dev Immunol. 2012;2012:284104. doi: 10.1155/2012/284104. Epub 2012 May 14. Clin Dev Immunol. 2012. PMID: 22666282 Free PMC article.
References
- Fontenot JD, Rudensky AY. Nat. Immunol. 2005;6:331. - PubMed
- Sakaguchi S, et al. Immunol. Rev. 2006;212:8. - PubMed
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. Nature. 2002;420:502. - PubMed
- Belkaid Y, Rouse BT. Nat. Immunol. 2005;6:353. - PubMed
- Rouse BT, Sarangi PP, Suvas S. Immunol. Rev. 2006;212:272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases