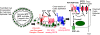Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts - PubMed (original) (raw)
Purinergic signaling in the lumen of a normal nephron and in remodeled PKD encapsulated cysts
Michael B Hovater et al. Purinergic Signal. 2008 Jun.
Abstract
The nephron is the functional unit of the kidney. Blood and plasma are continually filtered within the glomeruli that begin each nephron. Adenosine 5' triphosphate (ATP) and its metabolites are freely filtered by each glomerulus and enter the lumen of each nephron beginning at the proximal convoluted tubule (PCT). Flow rate, osmolality, and other mechanical or chemical stimuli for ATP secretion are present in each nephron segment. These ATP-release stimuli are also different in each nephron segment due to water or salt permeability or impermeability along different luminal membranes of the cells that line each nephron segment. Each of the above stimuli can trigger additional ATP release into the lumen of a nephron segment. Each nephron-lining epithelial cell is a potential source of secreted ATP. Together with filtered ATP and its metabolites derived from the glomerulus, secreted ATP and adenosine derived from cells along the nephron are likely the principal two of several nucleotide and nucleoside candidates for renal autocrine and paracrine ligands within the tubular fluid of the nephron. This minireview discusses the first principles of purinergic signaling as they relate to the nephron and the urinary bladder. The review discusses how the lumen of a renal tubule presents an ideal purinergic signaling microenvironment. The review also illustrates how remodeled and encapsulated cysts in autosomal dominant polycystic kidney disease (ADPKD) and remodeled pseudocysts in autosomal recessive PKD (ARPKD) of the renal collecting duct likely create an even more ideal microenvironment for purinergic signaling. Once trapped in these closed microenvironments, purinergic signaling becomes chronic and likely plays a significant epigenetic and detrimental role in the secondary progression of PKD, once the remodeling of the renal tissue has begun. In PKD cystic microenvironments, we argue that normal purinergic signaling within the lumen of the nephron provides detrimental acceleration of ADPKD once remodeling is complete.
Figures
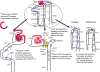
Fig. 1
Nucleotide secretion along the nephron: current postulates. Although not pictured as such, the sources of secreted ATP along the nephron are the renal epithelial cells themselves that line each nephron segment
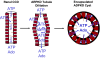
Fig. 2
Purinergic signaling in an encapsulated ADPKD cyst. The progression of remodeling in ARPKD versus ADPKD is shown in diagram format. Whereas some longer-lived extracellular ATP and adenosine (Ado) signaling may occur in dilated ARPKD segments, ADPKD cysts have lost communication with the rest of the nephron when encapsulated
Fig. 3
Postulated detrimental roles of purinergic signaling in an ARPKD pseudocyst or ADPKD cyst. Although ARPKD pseudocysts are not fully encapsulated, they may close off periodically due to limits in space between noncystic and cystic tissue parenchyma, leading to similar problems that occur in ADPKD encapsulated cysts. Adenosine stimulation of cyclic AMP and cystic fibrosis transmembrane-conductance-regulator (CFTR)-driven salt and water secretion and ATP stimulation of calcium-driven salt and water secretion are pictured, and both may proceed in parallel to the detriment of volume expansion in the cysts
Similar articles
- ATP release mechanisms, ATP receptors and purinergic signalling along the nephron.
Schwiebert EM. Schwiebert EM. Clin Exp Pharmacol Physiol. 2001 Apr;28(4):340-50. doi: 10.1046/j.1440-1681.2001.03451.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11339211 Review. - Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals.
Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Hovater MB, et al. Purinergic Signal. 2008 Jun;4(2):155-70. doi: 10.1007/s11302-007-9072-0. Epub 2007 Nov 13. Purinergic Signal. 2008. PMID: 18368523 Free PMC article. - ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys.
Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. Wilson PD, et al. J Am Soc Nephrol. 1999 Feb;10(2):218-29. doi: 10.1681/ASN.V102218. J Am Soc Nephrol. 1999. PMID: 10215320 - Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease.
Rangan G. Rangan G. Front Physiol. 2013 Aug 16;4:218. doi: 10.3389/fphys.2013.00218. eCollection 2013. Front Physiol. 2013. PMID: 23966953 Free PMC article. - Functional and therapeutic importance of purinergic signaling in polycystic kidney disease.
Ilatovskaya DV, Palygin O, Staruschenko A. Ilatovskaya DV, et al. Am J Physiol Renal Physiol. 2016 Dec 1;311(6):F1135-F1139. doi: 10.1152/ajprenal.00406.2016. Epub 2016 Sep 21. Am J Physiol Renal Physiol. 2016. PMID: 27654892 Free PMC article. Review.
Cited by
- Flow regulation of endothelin-1 production in the inner medullary collecting duct.
Pandit MM, Inscho EW, Zhang S, Seki T, Rohatgi R, Gusella L, Kishore B, Kohan DE. Pandit MM, et al. Am J Physiol Renal Physiol. 2015 Mar 15;308(6):F541-52. doi: 10.1152/ajprenal.00456.2014. Epub 2015 Jan 13. Am J Physiol Renal Physiol. 2015. PMID: 25587122 Free PMC article. - Lipid Peroxidation Drives Renal Cyst Growth In Vitro through Activation of TMEM16A.
Schreiber R, Buchholz B, Kraus A, Schley G, Scholz J, Ousingsawat J, Kunzelmann K. Schreiber R, et al. J Am Soc Nephrol. 2019 Feb;30(2):228-242. doi: 10.1681/ASN.2018010039. Epub 2019 Jan 3. J Am Soc Nephrol. 2019. PMID: 30606785 Free PMC article. - Basal release of ATP: an autocrine-paracrine mechanism for cell regulation.
Corriden R, Insel PA. Corriden R, et al. Sci Signal. 2010 Jan 12;3(104):re1. doi: 10.1126/scisignal.3104re1. Sci Signal. 2010. PMID: 20068232 Free PMC article. Review. - Strategies targeting cAMP signaling in the treatment of polycystic kidney disease.
Torres VE, Harris PC. Torres VE, et al. J Am Soc Nephrol. 2014 Jan;25(1):18-32. doi: 10.1681/ASN.2013040398. Epub 2013 Dec 12. J Am Soc Nephrol. 2014. PMID: 24335972 Free PMC article. Review. - Purinergic signalling in the kidney in health and disease.
Burnstock G, Evans LC, Bailey MA. Burnstock G, et al. Purinergic Signal. 2014 Mar;10(1):71-101. doi: 10.1007/s11302-013-9400-5. Epub 2013 Nov 22. Purinergic Signal. 2014. PMID: 24265071 Free PMC article. Review.
References
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10749727', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10749727/'}\]}
- Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr (2000) Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol 278:H1294–H1298 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11546651', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11546651/'}\]}
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ (2001) Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol 281:C1158–C1164 - PubMed
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '14766946', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/14766946/'}\]}
- Oleaeczyk JJ, Ellsworth ML, Stephenson AH, Linigro AJ, Sprague RS (2004) Nitric oxide inhibits ATP release from erythrocytes. J Pharmacol Exp Ther 309:1079–1084 - PubMed
- None
- Guyton AC, Hall JE (2000) Textbook of medical physiology, 10th edn. WB Saunders, Philadelphia, PA
- None
- Johnson LR (2003) Essential medical physiology, 3rd edn. Elsevier Academic Press, New York, NY
LinkOut - more resources
Full Text Sources