A role for sex chromosome complement in the female bias in autoimmune disease - PubMed (original) (raw)
A role for sex chromosome complement in the female bias in autoimmune disease
Deborah L Smith-Bouvier et al. J Exp Med. 2008.
Abstract
Most autoimmune diseases are more common in women than in men. This may be caused by differences in sex hormones, sex chromosomes, or both. In this study, we determined if there was a contribution of sex chromosomes to sex differences in susceptibility to two immunologically distinct disease models, experimental autoimmune encephalomyelitis (EAE) and pristane-induced lupus. Transgenic SJL mice were created to permit a comparison between XX and XY within a common gonadal type. Mice of the XX sex chromosome complement, as compared with XY, demonstrated greater susceptibility to both EAE and lupus. This is the first evidence that the XX sex chromosome complement, as compared with XY, confers greater susceptibility to autoimmune disease.
Figures
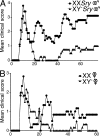
Figure 1.
The XX sex chromosome complement, as compared with the XY−, confers greater disease severity to active EAE. (A) Active EAE was induced in castrated XX_Sry_ and XY−Sry male mice with autoantigen PLP 139–151. Mean clinical disease course was more severe in castrated male XX_Sry_ mice as compared with XY−Sry mice. P < 0.0001. XX_Sry_, •, n = 6; XY−Sry, ▴, n = 5. (B) Active EAE was induced in ovariectomized XX and XY− female mice with autoantigen PLP 139–151. Clinical disease course was more severe in ovariectomized female XX mice compared with XY− mice. P < 0.0001. XX, •, n = 5; XY−, ▴, n = 5. Data are representative of one experiment in males and two independent experiments in females. Graphs show mean clinical score for each group at each time point. Statistical analysis compares mean score for all days from each group. Female symbol with x overlay indicates ovariectomized female; male symbol with x overlay indicates castrated male.
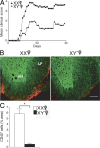
Figure 2.
The XX sex chromosome complement, as compared with the XY−, confers greater disease severity to adoptive EAE. (A) Effect of sex chromosome complement on adoptive EAE. LNCs from ovariectomized female XX and XY− mice (immunized with PLP 139–151) were adoptively transferred into WT females (gonadally intact). Mean clinical disease course was significantly higher in recipients of LNCs derived from XX mice as compared with those derived from XY− mice. P < 0.0001. XX, •, n = 13; XY−, ▴, n = 8. Data are representative of two independent experiments. Graph shows mean clinical score for each group at each time point. Statistical analysis compares mean score for all days from each group. (B) Recipients of XX-derived LNCs had more CNS inflammation than recipients of XY−-derived LNCs. Shown are representative thoracic spinal cord sections of mice with adoptively transferred EAE that were coimmunostained with anti-CD45 (red) and anti–β3-tubulin (green) antibodies. LF, lateral funiculus; VH, ventral horn). EAE mice that received LNCs derived from XX mice (left) had significantly increased CD45+ staining as compared with EAE mice that received LNCs derived from XY− mice (right). Bar, 100 μm. (C) Quantification of EAE neuropathology. Mice that received XX LNCs had a significant increase in CD45+ cells compared with mice that received XY− LNCs. P < 0.001, Student's t test. XX, •, n = 12; XY−, ▴, n = 12. Data are representative of two independent experiments. Histograms show the means and the SEM for mice in each group. Female symbol with x overlay indicates ovariectomized female.
Figure 3.
No effect of sex chromosome complement for active EAE in C57BL/6 mice. (A) Active EAE was induced in castrated male XX_Sry_ and XY−Sry C57BL/6 mice with autoantigen MOG 35–55. Mean clinical disease course was no different when comparing castrated male XX_Sry_ mice to XY−Sry mice. XX_Sry_, •, n = 6; XY−Sry, ▴, n = 6. (B) Active EAE was induced in ovariectomized female XX and XY− C57BL/6 mice with autoantigen MOG 35–55. Mean clinical disease course was also no different when comparing ovariectomized female XX versus XY− mice. XX, •, n = 6; XY−, ▴, n = 4. Data are representative of three independent experiments in males and two independent experiments in females. Graphs show mean clinical score for each group at each time point. Statistical analysis compares mean score for all days from each group. Female symbol with x overlay indicates ovariectomized female; male symbol with x overlay indicates castrated male.
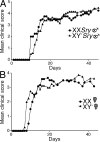
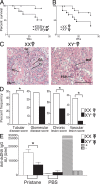
Figure 4.
The XX sex chromosome complement, as compared with the XY−, confers greater disease severity to lupus. (A) Castrated male XX_Sry_ and XY−Sry SJL mice were injected with pristane and monitored daily for signs of disease. By 26 wk, XX_Sry_ mice had increased mortality as compared with XY−Sry mice. P = 0.027. XX_Sry_, •, n = 12; XY−Sry, ▴, n = 13. (B) Ovariectomized female XX and XY− SJL mice were injected with pristane and monitored daily for signs of disease. At 26 wk, XX mice had increased mortality as compared with XY− mice. P = 0.036. XX, •, n = 13; XY−, ▴, n = 15. Data are representative of two independent experiments. (C) Kidneys from ovariectomized female XX and XY− female SJL mice injected with pristane were harvested upon death for renal pathology. Representative renal histology from pristane-induced lupus SJL mice showing that ovariectomized female XX mice (left) have more severe nephritis than ovariectomized female XY− mice (right). GI, glomerular infiltration; GS, segmental glomerulosclerosis; Cr, cellular crescent; TC, tubular casts; TA, tubular atrophy; FSP, focal segmental proliferative; MM, mild mesangial matrix. Bars, 50 μm. (D) Lesions from pristane-injected ovariectomized female XX and XY− mice were scored, ranked, and expressed as the percent frequency of mice in each group. A greater percentage of XX mice showed severe tubular disease scores (≥10; P = 0.006), chronic lesion scores (≥3; P = 0.046), and vascular lesion scores (P = 0.013) than their XY− littermates. In addition, a greater percentage of XX mice showed severe glomerular disease scores (≥10; NS). XX, n = 13; XY−, n = 14. XX, white columns; XY−, shaded columns. (E) Effect of sex chromosome complement on autoantibody levels in pristane-induced lupus. Ovariectomized female XX and XY− SJL mice were bled to detect anti-dsDNA antibodies in serum. 16 wk after pristane injection, XX mice had significantly higher levels of anti-dsDNA IgG antibody in sera than XY− mice. P < 0.01. XX, n = 9, white columns; XY−, n = 9, shaded columns; (NZBxNZW)F1 (+control), diagonal-lined columns; BALB/c (−control), vertical-lined columns. Histograms show the means and the SEM for mice in each group from one of three independent experiments. Female symbol with x overlay indicates ovariectomized female; male symbol with x overlay indicates castrated male.
Figure 5.
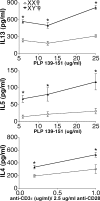
Effect of sex chromosome complement on Th2 cytokine levels. Ovariectomized female XX and XY− SJL mice were immunized with PLP 139–151, and after 10 d, draining LNCs were stimulated with PLP 139–151 and assessed for cytokine levels in supernatants. IL-13 (top) and IL-5 (middle) were significantly increased in XY− females compared with XX females. *, P < 0.05. XX, •, n = 4; XY−, ▴, n = 3. Ovariectomized female XX and XY− SJL mice were injected with pristane, and after 10 d, splenocytes were stimulated with anti-CD3ε/anti-CD28 and cultured for cytokine production. IL-4 levels in supernatant were significantly higher in XY− females as compared with XX females. *, P < 0.05. XX, •, n = 3; XY−, ▴, n = 3. All data are representative of at least three independent experiments. Graphs show the means and the SEM of mice in each group for each concentration. Female symbol with x overlay indicates ovariectomized female.
Figure 6.
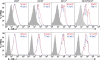
Effect of sex chromosome complement on IL-13Rα2 expression. Freshly isolated splenocytes from PLP 139–151 immunized SJL mice of the XX or the XY− sex chromosome complement were analyzed for the expression of IL-13Rα2 (top) and IL-13Rα1 (bottom) on B cells (CD19+), macrophages (CD11b+), dendritic cells (CD11c+), and myeloid dendritic cells (CD11c+CD11b+) by flow cytometry. XX, thick red lines; XY−, blue lines; shaded area, negative control. The mean fluorescent intensities are indicated on FACS plots (red, XX; blue, XY−). IL-13Rα2 expression was significantly higher on macrophages, dendritic cells, and myeloid dendritic cells in XX mice than in XY− mice. *, P < 0.05; n = 3 each. Results represent two independent experiments.
Similar articles
- Testosterone deficiency promotes arterial stiffening independent of sex chromosome complement.
Sakamuri A, Visniauskas B, Kilanowski-Doroh I, McNally AB, Imulinde A, Kamau A, Sengottaian D, McLachlan J, Anguera M, Mauvais-Jarvis F, Lindsey SH, Ogola BO. Sakamuri A, et al. Biol Sex Differ. 2024 Jun 6;15(1):46. doi: 10.1186/s13293-024-00624-0. Biol Sex Differ. 2024. PMID: 38845040 Free PMC article. - XX sex chromosome complement modulates immune responses to heat-killed Streptococcus pneumoniae immunization in a microbiome-dependent manner.
Amato-Menker CJ, Hopen Q, Pettit A, Gandhi J, Hu G, Schafer R, Franko J. Amato-Menker CJ, et al. Biol Sex Differ. 2024 Mar 14;15(1):21. doi: 10.1186/s13293-024-00597-0. Biol Sex Differ. 2024. PMID: 38486287 Free PMC article. - XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis.
Du S, Itoh N, Askarinam S, Hill H, Arnold AP, Voskuhl RR. Du S, et al. Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2806-11. doi: 10.1073/pnas.1307091111. Epub 2014 Feb 3. Proc Natl Acad Sci U S A. 2014. PMID: 24550311 Free PMC article. - The X in sex: how autoimmune diseases revolve around sex chromosomes.
Selmi C. Selmi C. Best Pract Res Clin Rheumatol. 2008 Oct;22(5):913-22. doi: 10.1016/j.berh.2008.09.002. Best Pract Res Clin Rheumatol. 2008. PMID: 19028371 Review. - Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes?
Zore T, Palafox M, Reue K. Zore T, et al. Mol Metab. 2018 Sep;15:35-44. doi: 10.1016/j.molmet.2018.04.003. Epub 2018 Apr 12. Mol Metab. 2018. PMID: 29706320 Free PMC article. Review.
Cited by
- Sex as a biological variable in ageing: insights and perspectives on the molecular and cellular hallmarks.
Fritz García JHG, Keller Valsecchi CI, Basilicata MF. Fritz García JHG, et al. Open Biol. 2024 Oct;14(10):240177. doi: 10.1098/rsob.240177. Epub 2024 Oct 30. Open Biol. 2024. PMID: 39471841 Free PMC article. Review. - Female-bias in systemic lupus erythematosus: How much is the X chromosome to blame?
Vieira AA, Almada-Correia I, Inácio J, Costa-Reis P, da Rocha ST. Vieira AA, et al. Biol Sex Differ. 2024 Oct 7;15(1):76. doi: 10.1186/s13293-024-00650-y. Biol Sex Differ. 2024. PMID: 39375734 Free PMC article. Review. - Sex difference in human diseases: mechanistic insights and clinical implications.
Shi Y, Ma J, Li S, Liu C, Liu Y, Chen J, Liu N, Liu S, Huang H. Shi Y, et al. Signal Transduct Target Ther. 2024 Sep 10;9(1):238. doi: 10.1038/s41392-024-01929-7. Signal Transduct Target Ther. 2024. PMID: 39256355 Free PMC article. Review. - Sex chromosomes and hormones independently influence healthy brain development but act similarly after cranial radiation.
Yeung J, DeYoung T, Spring S, de Guzman AE, Elder MW, Beauchamp A, Wong CS, Palmert MR, Lerch JP, Nieman BJ. Yeung J, et al. Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2404042121. doi: 10.1073/pnas.2404042121. Epub 2024 Aug 29. Proc Natl Acad Sci U S A. 2024. PMID: 39207735 - Neurologic Care for Transgender and Gender-Diverse People: A Review of Current Evidence and Clinical Implications.
Zeigler G, Harrington CA, Rosendale N, Ganos C, Roldan V, Pace A, Alick-Lindstrom S, Orozco-Poore C, Deeb W, Hansen ML, L'Erario ZP. Zeigler G, et al. Neurol Clin Pract. 2024 Oct;14(5):e200332. doi: 10.1212/CPJ.0000000000200332. Epub 2024 Jun 18. Neurol Clin Pract. 2024. PMID: 38919931 Review.
References
- Whitacre, C.C., S.C. Reingold, and P.A. O'Looney. 1999. A gender gap in autoimmunity. Science. 283:1277–1278. - PubMed
- Capel, B., K.H. Albrecht, L.L. Washburn, and E.M. Eicher. 1999. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech. Dev. 84:127–131. - PubMed
- Arnold, A.P. 2004. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 5:701–708. - PubMed
- Arnold, A.P., and P.S. Burgoyne. 2004. Are XX and XY brain cells intrinsically different? Trends Endocrinol. Metab. 15:6–11. - PubMed
- Voskuhl, R.R., H. Pitchekian-Halabi, A. MacKenzie-Graham, H.F. McFarland, and C.S. Raine. 1996. Gender differences in autoimmune demyelination in the mouse: implications for multiple sclerosis. Ann. Neurol. 39:724–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1T32 AR053463/AR/NIAMS NIH HHS/United States
- R01 AI050839/AI/NIAID NIH HHS/United States
- AI50839/AI/NIAID NIH HHS/United States
- R055057-01S1/PHS HHS/United States
- CA1028/CA/NCI NIH HHS/United States
- R01 AR050797/AR/NIAMS NIH HHS/United States
- NS043196/NS/NINDS NIH HHS/United States
- AR50797/AR/NIAMS NIH HHS/United States
- AR47322/AR/NIAMS NIH HHS/United States
- R01 NS043196/NS/NINDS NIH HHS/United States
- R21 AI070306/AI/NIAID NIH HHS/United States
- U01 AR055057/AR/NIAMS NIH HHS/United States
- AI070306/AI/NIAID NIH HHS/United States
- R01 AR047322/AR/NIAMS NIH HHS/United States
- T32 AR053463/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases