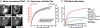Mathematical modeling identifies Smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system - PubMed (original) (raw)
Mathematical modeling identifies Smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system
Bernhard Schmierer et al. Proc Natl Acad Sci U S A. 2008.
Abstract
TGF-beta-induced Smad signal transduction from the membrane into the nucleus is not linear and unidirectional, but rather a dynamic network that couples Smad phosphorylation and dephosphorylation through continuous nucleocytoplasmic shuttling of Smads. To understand the quantitative behavior of this network, we have developed a tightly constrained computational model, exploiting the interplay between mathematical modeling and experimental strategies. The model simultaneously reproduces four distinct datasets with excellent accuracy and provides mechanistic insights into how the network operates. We use the model to make predictions about the outcome of fluorescence recovery after photobleaching experiments and the behavior of a functionally impaired Smad2 mutant, which we then verify experimentally. Successful model performance strongly supports the hypothesis of a dynamic maintenance of Smad nuclear accumulation during active signaling. The presented work establishes Smad nucleocytoplasmic shuttling as a dynamic network that flexibly transmits quantitative features of the extracellular TGF-beta signal, such as its duration and intensity, into the nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
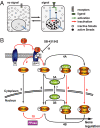
Fig. 1.
Smad nucleocytoplasmic dynamics. (A) A cartoon of Smad nucleocytoplasmic dynamics. Inactive Smads shuttle between the nucleus and cytoplasm. Receptor-activated Smads reside preferentially in the nucleus and accumulate there. Slow inactivation of active Smads in the nucleus, however, continuously releases inactivated Smads into the cytoplasm. In the presence of a signal, reactivation of cytoplasmic Smads dynamically maintains nuclear accumulation. As soon as receptor activity ceases, this mechanism restores the system back to the uninduced state. (B) Network topology used for model construction. Red arrows indicate irreversible reactions. Black arrows indicate reversible reactions. TGF-β binding converts inactive receptors (R) irreversibly into active receptors (Ract) with a rate constant _k_TGFβ (1). Active receptors irreversibly phosphorylate cytoplasmic Smad2 with a rate constant _k_phos (2). In both the nucleus and the cytoplasm, phospho-Smad2 forms heteromeric complexes with Smad4 (3A and 3B) and homomeric complexes (4A and 4B). These reversible reactions are described by the off-rate _k_off and the dissociation constant _K_diss. Smad2 (5), monomeric phospho-Smad2 (6), and Smad4 (7) shuttle reversibly between nucleus and cytoplasm with the rate constants _k_in and _k_ex. Heteromeric complexes (8) and homomeric complexes (9) are imported into the nucleus with a rate constant _k_in times CIF (complex import factor), but cannot be exported. The Smad2 phosphatase (PPase) is nuclear and irreversibly dephosphorylates monomeric phospho-Smad2 with a rate constant _k_dephos (10). Finally, the receptor kinase is reversibly blocked by the inhibitor SB-431542 (11), which is described by the interaction's off-rate, _k_offSB, and dissociation constant, _K_dissSB. For further details see
SI Text
and
Fig. S2
.
Fig. 2.
The RECI model is superior in fitting experimental datasets. (A) Live cell imaging in HaCaT EGFP-Smad2 cells in response to TGF-β and SB-431542 yielded Dataset 1 and Dataset 2, the kinetics of Smad2 nuclear accumulation and nuclear Smad2 clearance, respectively. Images of a subset of the sampled time course are shown. (B) Dataset 3. Kinetics of TGF-β-induced Smad2 phosphorylation. Representative immunoblots probed with anti-phospho-Smad2 and anti-Smad2/3 antibodies are shown. (C) Dataset 4. The nucleocytoplasmic distribution of total phospho-Smad2 immunoreactivity. Cytoplasmic (cyt) and total extracts (tot) were immunoblotted as in B. Double amounts of cytoplasmic extracts were loaded compared with total cell extracts. (D) Both models fit the average nuclear accumulation and average nuclear clearance data equally well. Images as in A were analyzed by measuring nuclear fluorescence (○; ± SD, n = 10 cells). The black line is the RO model fit, and the red line is RECI model fit. (E) Experimental phosphorylation data from immunoblots as shown in B (○; ± SD, n = 3). The black line and black ordinate indicate RO model fit. The red line and red ordinate indicate RECI model fit. The RECI model is superior in fitting the phosphorylation data and predicts a lesser extent of Smad2 phosphorylation. (F) Quantification of the levels of phosphorylated EGFP-Smad2 and endogenous Smad2 in the cytoplasm at maximal response to TGF-β derived from experiments like those shown in C compared with the predicted levels from the RO and RECI models. In contradiction to the experimental value, the RO model predicts high levels of cytoplasmic phospho-Smad2. The RECI model agrees well with the experimental value. (G) The best-fit parameter sets for the RECI and RO models. For a detailed description see
SI Text
.
Fig. 3.
The RECI model correctly recapitulates a FRAP experiment. (A) After 1 h of TGF-β treatment, nuclear EGFP-Smad2 was photobleached and nuclear fluorescence recovery was monitored. Representative pictures of a recovery time course are shown. (B) To model the FRAP recovery, simulations were run for both models until maximal response to TGF-β was reached. The simulation was stopped, and from these initial conditions (RECI model: 68 nM EGFP-Smad2 in the nucleus, 42 nM in the cytoplasm; RO model: 64 nM in the nucleus, 45 nM in the cytoplasm), a photobleach was simulated by converting all nuclear species containing EGFP-Smad2 into the corresponding species containing endogenous, i.e., “invisible” Smad2. The simulation was continued, and the sum of the concentrations of all nuclear fluorescent species was plotted. Because the experimental data were measured in arbitrary units, a least-square method was used to scale the experimental dataset (○; ± SD, n = 10) such that maximal overlap with either the RECI model (red line and red ordinate) or the RO model (black line, black ordinate) was achieved. Note the superior performance of the RECI model when compared with the RO model in capturing the shape of the recovery curve. (C) The recovery curve (red line) is a sum of the recovery behavior of all species containing EGFP-Smad2, whose concentrations were calculated by using the RECI model.
Fig. 4.
The RECI model correctly predicts the behavior of a mutant Smad2 unable to form Smad complexes. (A) EGFP-Smad2 D300H is predicted to be phosphorylated and dephosphorylated with highly similar kinetics to endogenous Smad2. (B) Representative immunoblots showing a time course of phosphorylation and dephosphorylation of EGFP-Smad2 D300H and of endogenous Smad2 in response to TGF-β and subsequent receptor inhibition. Total EGFP-Smad2 D300H and Smad2/3 are shown as loading controls. Note that EGFP-Smad2 D300H is expressed at lower levels compared with endogenous Smad2, and films showing EGFP-Smad2 D300H were exposed for a longer time to allow for direct comparison of the kinetics. The quantification shows that phosphorylation and dephosphorylation of mutant and wild-type Smad2 proceed with indistinguishable kinetics. (C) Phosphorylation and dephosphorylation kinetics of endogenous Smad2 and EGFP-Smad2 D300H assuming that 20% of the total phosphatase activity is cytoplasmic. Note the dramatic increase in the dephosphorylation rate of EGFP-Smad2 D300H on SB-431542 treatment compared with Smad2, differences that are not observed experimentally (B). (D) Even at full response, phosphorylated EGFP-Smad2 D300H is predicted to reside mainly in the cytoplasm. (E) Immunoblot of nuclear and cytoplasmic fractions of HaCaT EGFP-Smad2 cells and HaCaT EGFP-Smad2 D300H cells. Wild-type phosphorylated EGFP-Smad2 shows the same distribution as endogenous phospho-Smad2 (lanes 1–8). Phosphorylated EGFP-Smad2 D300H is enriched in the cytoplasm (lanes 9–16). Note that EGFP-Smad2 D300H HaCaT cells express lower levels of the transgene than EGFP-Smad2 HaCaT cells (7, 10). PARP and Grb-2 were used as fractionation and loading controls. The PARP antibody shows a cross-reaction with unrelated proteins of slightly lower mobility in the cytoplasm.
Fig. 5.
Predictions from the RECI model and parameter sensitivity analysis. (A) Calculated concentrations of Smad species in the nucleus and cytoplasm during TGF-β stimulation. Concentrations given refer to the sum of Smad2 and EGFP-Smad2. (B) As an input function, receptor activity was changed according to a double step function (blue line). The effect on the nuclear concentration of Smad2/Smad4 complexes (red line) was calculated. (C) As in B, except receptor activity is represented by a step function with added rapid fluctuations. (D) Sensitivity analysis. Scaled sensitivity coefficients for the plateau concentration of nuclear Smad2/Smad4 complexes to variations in the individual reaction rates are given. CIF, complex import factor for the respective complex (defined as import rate of Smad complexes over the import rate of monomeric Smads, see
SI Text
).
Similar articles
- Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads.
Schmierer B, Hill CS. Schmierer B, et al. Mol Cell Biol. 2005 Nov;25(22):9845-58. doi: 10.1128/MCB.25.22.9845-9858.2005. Mol Cell Biol. 2005. PMID: 16260601 Free PMC article. - Analysis of Smad nucleocytoplasmic shuttling in living cells.
Nicolás FJ, De Bosscher K, Schmierer B, Hill CS. Nicolás FJ, et al. J Cell Sci. 2004 Aug 15;117(Pt 18):4113-25. doi: 10.1242/jcs.01289. Epub 2004 Jul 27. J Cell Sci. 2004. PMID: 15280432 - Smad signaling dynamics: insights from a parsimonious model.
Shankaran H, Wiley HS. Shankaran H, et al. Sci Signal. 2008 Sep 9;1(36):pe41. doi: 10.1126/scisignal.136pe41. Sci Signal. 2008. PMID: 18780891 - Nucleocytoplasmic shuttling of Smad proteins.
Hill CS. Hill CS. Cell Res. 2009 Jan;19(1):36-46. doi: 10.1038/cr.2008.325. Cell Res. 2009. PMID: 19114992 Review. - Regulation of Smad activities.
Xu L. Xu L. Biochim Biophys Acta. 2006 Nov-Dec;1759(11-12):503-13. doi: 10.1016/j.bbaexp.2006.11.001. Epub 2006 Nov 15. Biochim Biophys Acta. 2006. PMID: 17182123 Free PMC article. Review.
Cited by
- Cooperative assembly of Co-Smad4 MH1 with R-Smad1/3 MH1 on DNA: a molecular dynamics simulation study.
Wang G, Li C, Wang Y, Chen G. Wang G, et al. PLoS One. 2013;8(1):e53841. doi: 10.1371/journal.pone.0053841. Epub 2013 Jan 10. PLoS One. 2013. PMID: 23326519 Free PMC article. - Nuclear Export of Smads by RanBP3L Regulates Bone Morphogenetic Protein Signaling and Mesenchymal Stem Cell Differentiation.
Chen F, Lin X, Xu P, Zhang Z, Chen Y, Wang C, Han J, Zhao B, Xiao M, Feng XH. Chen F, et al. Mol Cell Biol. 2015 May;35(10):1700-11. doi: 10.1128/MCB.00121-15. Epub 2015 Mar 9. Mol Cell Biol. 2015. PMID: 25755279 Free PMC article. - In silico and in vitro modeling of hepatocyte drug transport processes: importance of ABCC2 expression levels in the disposition of carboxydichlorofluroscein.
Howe K, Gibson GG, Coleman T, Plant N. Howe K, et al. Drug Metab Dispos. 2009 Feb;37(2):391-9. doi: 10.1124/dmd.108.022921. Epub 2008 Nov 20. Drug Metab Dispos. 2009. PMID: 19022944 Free PMC article. - Quantitative modeling and analysis of the transforming growth factor beta signaling pathway.
Chung SW, Miles FL, Sikes RA, Cooper CR, Farach-Carson MC, Ogunnaike BA. Chung SW, et al. Biophys J. 2009 Mar 4;96(5):1733-50. doi: 10.1016/j.bpj.2008.11.050. Biophys J. 2009. PMID: 19254534 Free PMC article. - Phospho-control of TGF-beta superfamily signaling.
Wrighton KH, Lin X, Feng XH. Wrighton KH, et al. Cell Res. 2009 Jan;19(1):8-20. doi: 10.1038/cr.2008.327. Cell Res. 2009. PMID: 19114991 Free PMC article. Review.
References
- Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. - PubMed
- Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. - PubMed
- Kavsak P, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-β receptor for degradation. Mol Cell. 2000;6:1365–1375. - PubMed
- Batut J, Howell M, Hill CS. Kinesin-mediated transport of Smad2 is required for signaling in response to TGF-β ligands. Dev Cell. 2007;12:261–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources