An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle - PubMed (original) (raw)
An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle
Christian Burgess et al. J Neurosci. 2008.
Abstract
Skeletal muscle tone is modulated in a stereotypical pattern across the sleep-wake cycle. Abnormalities in this modulation contribute to most of the major sleep disorders; therefore, characterizing the neurochemical substrate responsible for transmitting a sleep-wake drive to somatic motoneurons needs to be determined. Glutamate is an excitatory neurotransmitter that modulates motoneuron excitability; however, its role in regulating motoneuron excitability and muscle tone during natural sleep-wake behaviors is unknown. Therefore, we used reverse-microdialysis, electrophysiology, pharmacological, and histological methods to determine how changes in glutamatergic neurotransmission within the trigeminal motor pool contribute to the sleep-wake pattern of masseter muscle tone in behaving rats. We found that blockade of non-NMDA and NMDA glutamate receptors (via CNQX and d-AP-5) on trigeminal motoneurons reduced waking masseter tone to sleeping levels, indicating that masseter tone is maximal during alert waking because motoneurons are activated by an endogenous glutamatergic drive. This wake-related drive is switched off in non-rapid eye movement (NREM) sleep, and this contributes to the suppression of muscle tone during this state. We also show that a functional glutamatergic drive generates the muscle twitches that characterize phasic rapid-eye movement (REM) sleep. However, loss of a waking glutamatergic drive is not sufficient for triggering the motor atonia that characterizes REM sleep because potent activation of either AMPA or NMDA receptors on trigeminal motoneurons was unable to reverse REM atonia. We conclude that an endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone during wakefulness, NREM sleep, and phasic REM sleep but not during tonic REM sleep.
Figures
Figure 1.
Masseter muscle tone exhibits a stereotypical pattern of activity across the natural sleep–wake cycle. A, A typical example showing how different sleep–wake states affect basal levels of masseter and neck muscle tone. Masseter and neck muscle EMG activity are maximal in AW, reduced in QW, and further suppressed in NREM sleep. During tonic REM sleep, masseter and neck muscles are atonic except for flurries of muscle twitches that occur during periods of phasic REM sleep. B, Group data from 24 rats demonstrating that both masseter and neck muscle tone follow a stereotypical pattern of activity across the sleep–wake cycle, with muscle tone being significantly suppressed during both NREM and REM sleep (p < 0.001). Traces were taken during baseline conditions, before a microdialysis probe was inserted into the trigeminal motor pool. Data are expressed as mean percentage changes from alert waking. All values are means ± SEM.
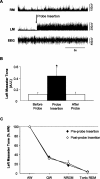
Figure 2.
Insertion of a microdialysis probe into the trigeminal motor pool has transient affects on left masseter muscle tone. A, A representative EMG trace showing that insertion of a microdialysis probe into the left trigeminal motor pool (during wakefulness) induced a transient activation of left masseter tone (LM); this intervention never affected right masseter (RM) EMG activity. B, Group data demonstrating that left masseter muscle tone significantly increased above baseline levels during probe insertion (p = 0.014); however, this affect only endured for 99 ± 50 s before it returned to baseline levels (p = 0.997). C, Group data demonstrate that the stereotypical pattern of left masseter muscle tone across the sleep cycle is unaffected by placing a probe in the trigeminal nucleus. Data are expressed as mean percentage changes from alert waking. All values are means ± SEM; *p < 0.05. A.U., Arbitrary units.
Figure 3.
Microdialysis probes were located in the left trigeminal motor nucleus. A, a is a photograph depicting a lesion made by a microdialysis probe in the trigeminal motor nucleus. b and c are sections that immediately flank the rostral and caudal borders of the trigeminal nucleus; there was no lesion in either area, demonstrating that the probe was located exclusively in the motor pool. Scale bars, 1 mm. B, Black filled circles represent the location of the lesions in the left trigeminal nuclei in the 28 rats used in these studies. Triangles represent the location of lesions not in the trigeminal motor nucleus; data from these two rats were not used in the study. C, A typical recording showing that perfusion of 0.1 m
m
AMPA into the left motor pool increases left masseter tone (LM) without affecting muscle activity in the right masseter (RM). D, Group data showing that AMPA perfusion induced a potent increase in left (p = 0.012) but not right masseter tone (p = 0.574). All values are means ± SEM; *p < 0.05. A.U., Arbitrary units.
Figure 4.
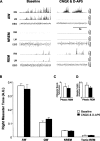
Antagonism of non-NMDA and NMDA receptors on trigeminal motoneurons in the left motor pool suppresses left but not right masseter tone. A, A typical example demonstrating that application of CNQX and
d
-AP-5 into the left trigeminal motor pool suppresses left masseter muscle tone during alert waking and REM sleep, without affecting right masseter muscle activity. B–D, Group data (n = 6) comparing right masseter muscle tone before (i.e., baseline) and after perfusion of CNQX and
d
-AP-5 into the left trigeminal motor pool. Although non-NMDA and NMDA receptor antagonism suppressed left masseter tone (see Fig. 5) during alert and quiet waking and reduced muscle twitch activity during REM sleep, this intervention had no affect on right masseter muscle tone during any behavioral state. These observations demonstrate that manipulations in the left motor pool do not affect motoneurons in the right trigeminal motor pool. All values are means ± SEM; A.U., arbitrary units.
Figure 5.
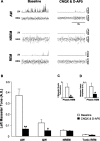
An endogenous glutamatergic drive onto trigeminal motoneurons is functional during wakefulness and REM sleep. A, A typical example showing that antagonism of non-NMDA and NDMA receptors on trigeminal motoneurons in the left motor pool reduces basal tone of left masseter muscle during alert waking; this intervention also abolishes the muscle twitches of phasic REM sleep. B, Group data demonstrating that application of CNQX and
d
-AP-5 caused a significant reduction in left masseter activity during alert and quiet waking but had no affect on basal masseter tone during either NREM or tonic REM sleep. C, D, Antagonism of non-NDMA and NMDA receptors decreased the number of muscle twitches in the left masseter muscle during phasic REM sleep and decreased the amplitude of the remaining muscle twitches. All values are means ± SEM; *p < 0.05 and **p < 0.001; A.U., arbitrary units.
Figure 6.
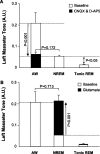
Withdrawal of a wake-related glutamatergic drive onto motoneurons contributes to the suppression of basal masseter muscle tone during NREM but not tonic REM sleep. A, Perfusion of CNQX and
d
-AP-5 into the trigeminal motor pool reduced waking masseter muscle tone to NREM sleep levels during baseline conditions (downward arrow; p < 0.001); however, this intervention was unable to reduce waking motor tone to tonic REM sleep levels during baseline conditions (i.e., significant difference between baseline tonic REM and AW during CNQX/
d
-AP-5, p < 0.05). B, Glutamate application during NREM sleep increased masseter muscle tone to waking levels during baseline conditions (p < 0.001); however, it did not increase muscle tone during tonic REM sleep (p = 0.916). All values are means ± SEM; A.U., arbitrary units.
Figure 7.
The functional glutamatergic drive onto trigeminal motoneurons during waking and phasic REM is mediated primarily by non-NMDA receptors. A, Group data (n = 6) demonstrating that antagonism of non-NMDA receptors by perfusion of 0.5 m
m
CNQX into the left trigeminal motor pool significantly decreased left masseter muscle tone during waking but had no effect on levels of masseter tone during either NREM (p = 0.939) or tonic REM (p = 0.985) sleep. B, C, This same intervention significantly decreased the number of muscle twitches during phasic REM sleep and the magnitude of the remaining muscle twitches. D, Group data (n = 5) demonstrating that antagonism of NMDA receptors by perfusion of 5.0 m
m d
-AP-5 into the left trigeminal motor pool significantly decreased left masseter muscle tone during waking by 49% but had no effect on levels of masseter tone during either NREM (p = 0.896) or tonic REM (p = 0.974) sleep. E, F, This same intervention did not decrease the number of muscle twitches during REM sleep (p = 0.231) or the amplitude of muscle twitches (p = 0.197). All values are means ± SEM; *p < 0.05 and **p < 0.001; A.U., arbitrary units.
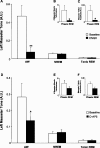
Figure 8.
Exogenous glutamate application at the trigeminal motor pool potently increases masseter muscle tone during all behavioral states except tonic REM sleep. A, A typical example showing that perfusion of glutamate into the left trigeminal motor pool potently increases left masseter muscle (LM) tone during both alert waking and during NREM sleep. Remarkably, glutamate application is unable to overcome the atonia of tonic REM sleep; however, it does increase the number of muscle twitches during phasic REM. B, Group data (n = 6) demonstrating that exogenous glutamate application significantly increased left masseter muscle tone in waking and NREM sleep (p < 0.002), without changing levels of motor tone during tonic REM sleep (p = 0.916). C, D, Perfusion of glutamate into the left trigeminal motor pool also increased the number of muscle twitches during phasic REM sleep, but there was no change in the amplitude of muscle twitches after glutamate application (p = 0.099). All values are means ± SEM; *p < 0.05 and **p < 0.002; A.U., arbitrary units.
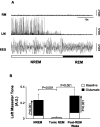
Figure 9.
The excitatory effects of glutamate are rapidly lost during entry into REM sleep but immediately regained in post-REM waking. A, A typical example showing that glutamate perfusion into the left trigeminal motor pool causes a potent activation of left masseter muscle (LM) activity during NREM sleep, but this excitatory effect is immediately abolished during entrance into REM sleep. Note that right masseter muscle (RM) tone is unaffected by glutamate application. B, Group data demonstrating that the excitatory effects of glutamate on trigeminal motoneurons are present in NREM sleep (glutamate vs baseline, p < 0.001) but rapidly lost on entry into tonic REM sleep (glutamate vs baseline, p = 0.916) and immediately regained during post-REM waking (glutamate vs baseline, p < 0.001). All values are means ± SEM; A.U., arbitrary units.
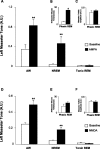
Figure 10.
AMPA and NMDA at the trigeminal motor pool increase masseter muscle tone during waking and NREM sleep but not during tonic REM sleep. A, Group data (n = 6) demonstrating that AMPA application significantly increased left masseter muscle tone in waking and NREM sleep, without changing levels of motor tone during tonic REM sleep (p = 0.879). B, C, During phasic REM sleep, AMPA application increased the number of muscle twitches without affecting twitch amplitude (p = 0.978). D, NMDA application also increased masseter muscle tone in waking and NREM sleep, without changing levels of muscle tone during tonic REM sleep (p = 0.895). E, F, During phasic REM sleep, NMDA application did not increase either the number (p = 0.192) or amplitude of muscle twitches (p = 0.688). All values are means ± SEM; **p < 0.003; A.U., arbitrary units.
Similar articles
- Noradrenergic modulation of masseter muscle activity during natural rapid eye movement sleep requires glutamatergic signalling at the trigeminal motor nucleus.
Schwarz PB, Mir S, Peever JH. Schwarz PB, et al. J Physiol. 2014 Aug 15;592(16):3597-609. doi: 10.1113/jphysiol.2014.272633. Epub 2014 May 23. J Physiol. 2014. PMID: 24860176 Free PMC article. - Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia.
Brooks PL, Peever JH. Brooks PL, et al. J Neurosci. 2008 Apr 2;28(14):3535-45. doi: 10.1523/JNEUROSCI.5023-07.2008. J Neurosci. 2008. PMID: 18385312 Free PMC article. - Noradrenaline triggers muscle tone by amplifying glutamate-driven excitation of somatic motoneurones in anaesthetized rats.
Schwarz PB, Yee N, Mir S, Peever JH. Schwarz PB, et al. J Physiol. 2008 Dec 1;586(23):5787-802. doi: 10.1113/jphysiol.2008.159392. Epub 2008 Oct 9. J Physiol. 2008. PMID: 18845613 Free PMC article. - Control of motoneuron function and muscle tone during REM sleep, REM sleep behavior disorder and cataplexy/narcolepsy.
Peever J. Peever J. Arch Ital Biol. 2011 Dec 1;149(4):454-66. doi: 10.4449/aib.v149i4.1257. Arch Ital Biol. 2011. PMID: 22205591 Review. - Confirmation of the consensus that glycinergic postsynaptic inhibition is responsible for the atonia of REM sleep.
Chase MH. Chase MH. Sleep. 2008 Nov;31(11):1487-91. doi: 10.1093/sleep/31.11.1487. Sleep. 2008. PMID: 19014068 Free PMC article.
Cited by
- Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity.
Horner RL. Horner RL. Philos Trans R Soc Lond B Biol Sci. 2009 Sep 12;364(1529):2553-64. doi: 10.1098/rstb.2009.0065. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19651656 Free PMC article. Review. - Noradrenergic modulation of masseter muscle activity during natural rapid eye movement sleep requires glutamatergic signalling at the trigeminal motor nucleus.
Schwarz PB, Mir S, Peever JH. Schwarz PB, et al. J Physiol. 2014 Aug 15;592(16):3597-609. doi: 10.1113/jphysiol.2014.272633. Epub 2014 May 23. J Physiol. 2014. PMID: 24860176 Free PMC article. - A Novel Bioengineered Functional Motor Unit Platform to Study Neuromuscular Interaction.
Saini J, Faroni A, Reid AJ, Mamchaoui K, Mouly V, Butler-Browne G, Lightfoot AP, McPhee JS, Degens H, Al-Shanti N. Saini J, et al. J Clin Med. 2020 Oct 10;9(10):3238. doi: 10.3390/jcm9103238. J Clin Med. 2020. PMID: 33050427 Free PMC article. - Contribution of Neurochemical Inputs to the Decrease of Motoneuron Excitability During Non-REM and REM Sleep: A Systematic Review.
Fenik VB. Fenik VB. Front Neurol. 2018 Jul 31;9:629. doi: 10.3389/fneur.2018.00629. eCollection 2018. Front Neurol. 2018. PMID: 30108546 Free PMC article. - The neurobiology of sleep.
Siegel JM. Siegel JM. Semin Neurol. 2009 Sep;29(4):277-96. doi: 10.1055/s-0029-1237118. Epub 2009 Sep 9. Semin Neurol. 2009. PMID: 19742406 Free PMC article. Review.
References
- Alessandri B, Landolt H, Langemann H, Gregorin J, Hall J, Gratzl O. Application of glutamate in the cortex of rats: a microdialysis study. Acta Neurochir Suppl. 1996;67:6–12. - PubMed
- Aserinsky E, Kleitman N. Regularly oc-curring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
- Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep. 1999;22:1087–1092. - PubMed
- Brooks PL, Peever JH. Biochemical control of airway motor neurons during rapid eye movement sleep. Adv Exp Med Biol. 2008;605:437–441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL041370/HL/NHLBI NIH HHS/United States
- R01 NS014610/NS/NINDS NIH HHS/United States
- HL41370/HL/NHLBI NIH HHS/United States
- NS14610/NS/NINDS NIH HHS/United States
- R37 NS014610/NS/NINDS NIH HHS/United States
- R01 HL041370/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources