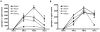Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection - PubMed (original) (raw)
Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection
Ting Jia et al. J Immunol. 2008.
Abstract
Chemokine receptor-mediated recruitment of inflammatory cells is essential for innate immune defense against microbial infection. Recruitment of Ly6C(high) inflammatory monocytes from bone marrow to sites of microbial infection is dependent on CCR2, a chemokine receptor that responds to MCP-1 and MCP-3. Although CCR2(-/-) mice are markedly more susceptible to Listeria monocytogenes infection than are wild-type mice, MCP-1(-/-) mice have an intermediate phenotype, suggesting that other CCR2 ligands contribute to antimicrobial defense. Herein, we show that L. monocytogenes infection rapidly induces MCP-3 in tissue culture macrophages and in serum, spleen, liver, and kidney following in vivo infection. Only cytosol invasive L. monocytogenes induce MCP-3, suggesting that cytosolic innate immune detection mechanisms trigger chemokine production. MCP-3(-/-) mice clear bacteria less effectively from the spleen than do wild-type mice, a defect that correlates with diminished inflammatory monocyte recruitment. MCP-3(-/-) mice have significantly fewer Ly6C(high) monocytes in the spleen and bloodstream, and increased monocyte numbers in bone marrow. MCP-3(-/-) mice, like MCP-1(-/-) mice, have fewer TNF- and inducible NO synthase-producing dendritic cells (Tip-DCs) in the spleen following L. monocytogenes infection. Our data demonstrate that MCP-3 and MCP-1 provide parallel contributions to CCR2-mediated inflammatory monocyte recruitment and that both chemokines are required for optimal innate immune defense against L. monocytogenes infection.
Figures
Figure 1. Kinetics of in vivo MCP-1 and MCP-3 expression following L. monocytogenes infection
C57BL/6 mice were infected intravenously with 3,000 live L. monocytogenes. At the indicated times, spleens, livers and kidneys were removed and serum was harvested and MCP-1 and MCP-3 levels were determined. (A) MCP-1and (B) MCP-3 levels in organ lysates and serum were quantified by sandwich ELISA. Each bar represents three mice and the experiment was repeated three times. Error bars show standard deviation.
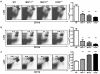
Figure 2. MCP-3 induction requires cytosol invasion by bacteria
(A) C57BL/6 mice were infected intravenously with 3,000 wild-type, 108 LLO-deficient L. monocytogenes, 5 × 105 ActA-deficient L. monocytogenes or immunized with 109 HKLM. Spleens were removed and lysed 24hr later. MCP-1 and MCP-3 levels in spleen lysates were quantified by ELISA. Each bar represents three mice and the experiment was repeated twice. Error bars show standard deviation. (B) BMMØ from C57BL/6 mice were infected with wild-type, LLO-deficient L. monocytogenes strains at a 5:1 ratio or cultured with 108/ml of HKLM. As a positive control, cells were cultured with 100ng/ml LPS. Supernatants were collected at indicated time and chemokine levels were quantified by ELISA. Each bar represents the average of three wells and this experiment was repeated twice.
Figure 3. MCP-3-/- mice are more susceptible to L. monocytogenes infection
C57BL/6, MCP-1-/-, MCP-3-/- and CCR2-/- mice were infected intravenously with 3,000 L. monocytogenes. Spleens were removed at (A) day 3 and (B) day 5, and viable bacteria were quantified. Mean numbers of colony forming units (CFU) from groups of 5-10 mice are shown. Error bars represent standard deviation, and this experiment was repeated twice. ** P<0.01 as compared to wild-type controls. Double dagger, number of dead animals/total in the group.
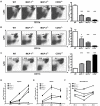
Figure 4. Homeostatic monocyte trafficking is impaired in the absence of MCP-3
(A) Spleen, (B) blood and (C) bone marrow were harvested from naïve C57BL/6, MCP-1-/-, MCP-3-/- and CCR2-/- mice. Cells were stained for CD11b and Ly6C. A large gate was drawn on the live lymphocyte/monocyte population and expression of CD11b and Ly6C was analyzed. Representative dot plots for three mice per group are shown on left. Percentages of CD11bint/Ly6Chigh cells in naïve wild-type, MCP-1-/-, MCP-3-/- and CCR2-/- spleens and circulation are shown on right. Each bar represents the average of 6-9 mice from two independent experiments. Error bars represent standard deviation. *p=0.012 **p<0.01 ***p<0.0001 as compared with wild-type controls.
Figure 5. MCP-3-mediated recruitment of Tip-DCs following infection
(A) Spleens, (B) blood and (C) bone marrows were harvested from C57BL/6, MCP-1-/-, MCP-3-/- and CCR2-/- mice infected intravenously with 3,000 L. monocytogenes 24hr and 48hr postinfection. Cells were stained for CD11b and Ly6C. A large gate was drawn on the live lymphocyte/monocyte population and expression of CD11b and Ly6C was analyzed. Representative dot plots for three mice per group are shown on the left. Percentages of CD11bint/Ly6Chigh cells in infected wild-type, MCP-1-/-, MCP-3-/- and CCR2-/- spleens, blood, and bone marrow are shown on the right. (D-F) Kinetics of CD11bint/Ly6Chigh population in wild-type, MCP-1-/-, MCP-3-/- and CCR2-/- spleens (D), blood (E), and bone marrows (F) following infection are shown. Each bar or time point represents the average of 9-12 mice from three independent experiments. Error bars represent standard deviation. *p=0.02 ***p<0.0001 as compared with wild-type controls.
Figure 6. MCP-3 is not required for Tip-DC development
Splenocytes from C57BL/6, MCP-1-/-, MCP-3-/- and CCR2-/- mice infected with 3,000 L. monocytogenes were collected 24hr postinfection for intracellular TNF staining, and 30hr postinfection for intracellular iNOS staining. Cells were gated on lymphocytes/monocytes, and expression of CD11b and TNF-α in the gate was analyzed. The percentages of (A) CD11bintTNF-αhi cells and (B) CD11bintiNOS+ cells are indicated. Representative dot plots for three mice per group are shown. The experiment was repeated twice with similar results.
Figure 7. Models of MCP-1 and MCP-3 functions in monocyte recruitment process
(A) Parallel model. Two populations of monocytes reside in different bone marrow niches, one responds to MCP-1 and the other responds to MCP-3. MCP-1 and MCP-3 are each responsible for one population of monocyte emigration from the bone marrow. (B) Series model. Monocyte emigration from bone marrow consist an inner bone marrow mobilization step and a bone marrow to circulation step. MCP-1 and MCP-3 each contributes to one step of this two-step process.
Similar articles
- MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection.
Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. Jia T, et al. J Immunol. 2009 Jul 15;183(2):1271-8. doi: 10.4049/jimmunol.0900460. Epub 2009 Jun 24. J Immunol. 2009. PMID: 19553532 Free PMC article. - Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression.
Shi C, Velázquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Shi C, et al. J Immunol. 2010 Jun 1;184(11):6266-74. doi: 10.4049/jimmunol.0904160. Epub 2010 Apr 30. J Immunol. 2010. PMID: 20435926 Free PMC article. - Monocyte-mediated immune defense against murine Listeria monocytogenes infection.
Serbina NV, Shi C, Pamer EG. Serbina NV, et al. Adv Immunol. 2012;113:119-34. doi: 10.1016/B978-0-12-394590-7.00003-8. Adv Immunol. 2012. PMID: 22244581 Free PMC article. Review. - Toxoplasma gondii profilin promotes recruitment of Ly6Chi CCR2+ inflammatory monocytes that can confer resistance to bacterial infection.
Neal LM, Knoll LJ. Neal LM, et al. PLoS Pathog. 2014 Jun 12;10(6):e1004203. doi: 10.1371/journal.ppat.1004203. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945711 Free PMC article. - Monocyte-mediated defense against microbial pathogens.
Serbina NV, Jia T, Hohl TM, Pamer EG. Serbina NV, et al. Annu Rev Immunol. 2008;26:421-52. doi: 10.1146/annurev.immunol.26.021607.090326. Annu Rev Immunol. 2008. PMID: 18303997 Free PMC article. Review.
Cited by
- Monocyte dysregulation: consequences for hepatic infections.
Sellau J, Puengel T, Hoenow S, Groneberg M, Tacke F, Lotter H. Sellau J, et al. Semin Immunopathol. 2021 Aug;43(4):493-506. doi: 10.1007/s00281-021-00852-1. Epub 2021 Apr 7. Semin Immunopathol. 2021. PMID: 33829283 Free PMC article. Review. - Radiation combined with thermal injury induces immature myeloid cells.
Mendoza AE, Neely CJ, Charles AG, Kartchner LB, Brickey WJ, Khoury AL, Sempowski GD, Ting JP, Cairns BA, Maile R. Mendoza AE, et al. Shock. 2012 Nov;38(5):532-42. doi: 10.1097/SHK.0b013e31826c5b19. Shock. 2012. PMID: 23042190 Free PMC article. - Monocytes from Irf5-/- mice have an intrinsic defect in their response to pristane-induced lupus.
Yang L, Feng D, Bi X, Stone RC, Barnes BJ. Yang L, et al. J Immunol. 2012 Oct 1;189(7):3741-50. doi: 10.4049/jimmunol.1201162. Epub 2012 Aug 29. J Immunol. 2012. PMID: 22933628 Free PMC article. - Small Noncoding RNA (sncRNA1) within the Latency-Associated Transcript Modulates Herpes Simplex Virus 1 Virulence and the Host Immune Response during Acute but Not Latent Infection.
Tormanen K, Matundan HH, Wang S, Jaggi U, Mott KR, Ghiasi H. Tormanen K, et al. J Virol. 2022 Apr 13;96(7):e0005422. doi: 10.1128/jvi.00054-22. Epub 2022 Mar 7. J Virol. 2022. PMID: 35254102 Free PMC article. - Deficiency of lymph node-resident dendritic cells (DCs) and dysregulation of DC chemoattractants in a malnourished mouse model of Leishmania donovani infection.
Ibrahim MK, Barnes JL, Osorio EY, Anstead GM, Jimenez F, Osterholzer JJ, Travi BL, Ahuja SS, White AC Jr, Melby PC. Ibrahim MK, et al. Infect Immun. 2014 Aug;82(8):3098-112. doi: 10.1128/IAI.01778-14. Epub 2014 May 12. Infect Immun. 2014. PMID: 24818662 Free PMC article.
References
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004;22:891–928. - PubMed
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL 63894/HL/NHLBI NIH HHS/United States
- R01 HL063894/HL/NHLBI NIH HHS/United States
- R37 AI039031/AI/NIAID NIH HHS/United States
- R01 HL052773/HL/NHLBI NIH HHS/United States
- R37 AI 03903/AI/NIAID NIH HHS/United States
- R01 AI080619/AI/NIAID NIH HHS/United States
- R01 HL 52773/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous