Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms - PubMed (original) (raw)
Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms
Andrew C Liu et al. PLoS Genet. 2008.
Abstract
The mammalian circadian clockwork is composed of a core PER/CRY feedback loop and additional interlocking loops. In particular, the ROR/REV/Bmal1 loop, consisting of ROR activators and REV-ERB repressors that regulate Bmal1 expression, is thought to "stabilize" core clock function. However, due to functional redundancy and pleiotropic effects of gene deletions, the role of the ROR/REV/Bmal1 loop has not been accurately defined. In this study, we examined cell-autonomous circadian oscillations using combined gene knockout and RNA interference and demonstrated that REV-ERBalpha and beta are functionally redundant and are required for rhythmic Bmal1 expression. In contrast, the RORs contribute to Bmal1 amplitude but are dispensable for Bmal1 rhythm. We provide direct in vivo genetic evidence that the REV-ERBs also participate in combinatorial regulation of Cry1 and Rorc expression, leading to their phase-delay relative to Rev-erbalpha. Thus, the REV-ERBs play a more prominent role than the RORs in the basic clock mechanism. The cellular genetic approach permitted testing of the robustness of the intracellular core clock function. We showed that cells deficient in both REV-ERBalpha and beta function, or those expressing constitutive BMAL1, were still able to generate and maintain normal Per2 rhythmicity. Our findings thus underscore the resilience of the intracellular clock mechanism and provide important insights into the transcriptional topologies underlying the circadian clock. Since REV-ERB function and Bmal1 mRNA/protein cycling are not necessary for basic clock function, we propose that the major role of the ROR/REV/Bmal1 loop and its constituents is to control rhythmic transcription of clock output genes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
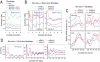
Figure 1. RORc activates Bmal1 transcription but is dispensable for Bmal1 rhythmicity.
(A) Circadian Bmal1 mRNA expression is blunted in the liver of Rorc −/− mice. The peak transcript levels of Bmal1, Clock, Npas2, and Cry1 are reduced in the liver of Rorc−/− mice compared to WT littermates, suggesting that RORc is an activator of Bmal1 transcription. Temporal patterns of Per2 and Dbp are unaltered. Expression was analyzed at 4-hr intervals by Q-PCR. Values are expressed as percentage of maximum expression for each gene. Error bars represent standard deviation (SD) of expression levels from four mice. Circadian time: hours after animal release in constant darkness. (B) Representative records of tissue-autonomous mPer2Luc bioluminescence rhythms in Rorc −/− lung explants. Rorc −/− lung explants displayed normal mPer2Luc rhythms, suggesting that Rorc is not required for circadian rhythmicity. Tissue explants were dissected and immediately cultured in explant medium for recording. Another medium change occurred at day 7. Circadian time: days after explant medium change. (C,D) Representative records of cell-autonomous bioluminescence rhythms in populations of Rorasg/sg fibroblasts transduced with a lentiviral Bmal1-dLuc reporter (C) or Per2-dLuc reporter (D). Rorasg/sg fibroblasts, in which no functional RORa, RORb, or RORc are expressed, displayed rhythmic oscillations of Bmal1-dLuc and Per2-dLuc reporters. Circadian time: days after explant medium change.
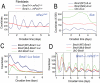
Figure 2. REV-ERBα and β are required for Bmal1 rhythms in fibroblasts.
(A) Representative bioluminescence rhythms from a Bmal1-dLuc reporter in fibroblasts deficient in either Rev-erbα or Rev-erbβ function. We tested two independent Rev-erbα−/− fibroblast cell lines and cells stably expressing an shRNA construct against Rev-erbβ. Fibroblasts deficient in either Rev-erbα or Rev-erbβ function alone displayed rhythmic oscillations of Bmal1-dLuc bioluminescence, suggesting functional redundancy of Rev-erbα and Rev-erbβ. Circadian time: days after explant medium change. (B) Representative bioluminescence patterns from a Bmal1-dLuc reporter in fibroblasts deficient in both Rev-erbα and Rev-erbβ function. Rev-erbα−/− fibroblasts stably expressing a non-specific control shRNA (shRNA-C) displayed circadian Bmal1-dLuc rhythms, but _Rev-erbα−/−:Rev-erbβ-_shRNA cells were arrhythmic, suggesting that the REV-ERBs are required for Bmal1 rhythmic expression. Three different shRNA constructs (shRNA-β1, β2 and β3) were used for knocking down endogenous Rev-erbβ expression in fibroblasts. Circadian time: days after explant medium change. (C) Temporal mRNA expression profiles of clock genes in Rev-erbα−/− fibroblasts stably expressing shRNA constructs against Rev-erbβ. Expression was analyzed at 2-hr intervals by Q-PCR. Values are expressed as percentage of maximum expression for each gene. Results were confirmed in two independent time courses. Similar results were obtained from both cell lines, and results for cell line-2 are presented here. For clarity, error bars representing SD of two culture samples for each cell line (<10%) were omitted. Rev-erbβ mRNA was significantly reduced by shRNA against Rev-erbβ, leading to higher expression levels of Bmal1 and Cry1. Per2 mRNA rhythms were unaltered in cells deficient in Rev-erbα and β function. Circadian time: hours after serum treatment. (D) Representative bioluminescence rhythms from a Per2-dLuc reporter in fibroblasts deficient in both Rev-erbα and Rev-erbβ function. Rev-erbα−/− fibroblasts expressing shRNA constructs against Rev-erbβ displayed Per2-dLuc rhythms similar to those of shRNA control cells. Similar results were obtained from all three shRNA constructs in two Rev-erbα−/− fibroblast cell lines, and results from cell line-2 are presented here. The three panels show patterns for the same cultures after three successive medium changes. Circadian time: days after explant medium change.
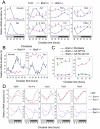
Figure 3. Cyclic expression of BMAL1 is not required for intracellular core clock function.
(A) Bioluminescence patterns of fibroblasts derived from WT and Bmal1−/−:mPer2Luc mice. Bmal1−/− fibroblasts are completely arrhythmic, suggesting that Bmal1 is required for clock function in fibroblasts. Circadian time: days after explant medium change. (B) Bioluminescence patterns in wild-type fibroblasts transduced with a lentiviral dLuc reporter. Bmal1(WT): Bmal1 promoter containing WT RORE sequence. Bmal1(Mut): Bmal1 promoter containing mutated RORE sequences. UbC: Ubiquitin C promoter. Unlike the Bmal1(WT), the Bmal1(Mut) and UbC promoters do not confer rhythmic luciferase expression. Circadian time: days after explant medium change. (C) Bioluminescence patterns in wild-type fibroblasts transduced with a lentiviral Bmal1::Luc fusion reporter. Unlike the Bmal1(WT), the Bmal1(Mut) and UbC promoters do not confer rhythmic BMAL1::LUC fusion protein expression. These results suggest that BMAL1 protein does not cycle and that only promoters that contain functional circadian elements confer rhythmic fusion protein expression. Circadian time: days after explant medium change. (D) Representative records of mPer2Luc rhythms in Bmal1−/− fibroblasts restored through genetic complementation. Lentiviral expression vectors carrying Bmal1 cDNA under control of different promoters were introduced into Bmal1−/−:mPer2Luc fibroblasts. The three promoters gave rise to similar levels of BMAL1 protein expression as determined by Q-PCR and Western blotting (data not shown). Both cyclically and constitutively expressed BMAL1 restored circadian rhythmicity in Bmal1−/− fibroblasts, suggesting that the rhythm of BMAL1 protein is not required for basic core clock function. Circadian time: days after explant medium change.
Figure 4. REV-ERBs play a prominent role in combinatorial regulation of Cry1 and Rorc.
(A) Temporal mRNA expression profiles of clock genes in the liver of Bmal1−/− mice. Expression was analyzed at 4-hr intervals by Q-PCR. Values are expressed as percentage of maximum expression for each gene. Error bar represents standard deviation (SD) of expression levels from four mice. The clock genes are presented in four groups based on different mRNA expression patterns (phase and level) in WT and Bmal1−/− mice. For instance, transcription of Cry1 and Rorc is elevated, rather than repressed, in the Bmal1−/− liver. Circadian time: hours after animal release in constant darkness. (B) Temporal mRNA expression profiles of Rev-erbα and Cry1 in Bmal1−/− fibroblasts. Expression was analyzed at 2-hr intervals by Q-PCR. Values are expressed as percentage of maximum expression for each gene. Results were confirmed in two independent time courses. Error bars represent SD of two culture samples for each cell line. Cry1 mRNA levels are constantly high throughout the day and Rev-erbα expression is completely abolished in Bmal1−/− fibroblasts, similar to results obtained from the liver. Circadian time: hours after serum treatment. (C) Over-expression (OX) of Rev-erbα represses elevated Cry1 mRNA levels in Bmal1−/− fibroblasts. Expression of GFP and REV-ERBα is driven by a constitutive CAG promoter. Temporal mRNA expression was analyzed at 3-hr intervals by Q-PCR. Values are expressed as percentage of maximum expression for each gene. Results were confirmed in two independent experiments. Error bars represent SD of two culture samples for each cell line. REV-ERBα expression was confirmed by Q-PCR, and also by Western blotting (data not shown). Circadian time: hours after serum treatment. (D) Temporal mRNA expression profiles of clock-controlled output genes in the liver of Rorc −/− and Bmal1−/− mice. Experiments were performed as described in Figure 1A for Rorc −/− mice and Figure 4A for Bmal1−/− mice. As for Bmal1 and Cry1, the prominent role of REV-ERBs in regulating transcription explains the elevated mRNA levels of these output genes in Bmal1−/− mice. For clarity, error bars representing SD from four mice (<10% for each gene) were omitted. Circadian time: hours after animal release in constant darkness.
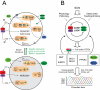
Figure 5. Model for circadian core clock mechanism and function.
(A) Different transcriptional regulation gives rise to differential phasing of clock genes. PER/CRY and BMAL1/CLOCK (BMAL/CLK) form the core feedback loop mediated by the E-box. The RORs and REV-ERBs are directly regulated by the core loop and provide additional positive and negative feedbacks, respectively, to Bmal1/Clock transcription. Four main types of gene regulatory mechanisms exist in a wild-type cell (top): 1) Rev-erbα and β (Rev) are driven primarily by E-box-mediated transcription, 2) Per1 and Per2 are regulated by BMAL1/CLOCK and additionally by a tonic signal input (T), 3) Cry1 and Rorc are regulated by BMAL1/CLOCK and ROR/REV as well as a tonic signal, and 4) Bmal1 and Clock are regulated by ROR/REV and a tonic signal. These different modes of transcriptional regulation provide the mechanistic basis for the different phases of their mRNAs (e.g. Rorc phase-delays Rev-erbα) in WT cells and the differential levels of expression (e.g., diminished REV leads to Cry1 up-regulation) in Bmal1−/− cells. BMAL1 is an essential clock component, and Bmal1−/− cells are completely arrhythmic (bottom). However, its rhythmic patterns of mRNA and protein expression are not required for core clock function. Genetic complementation by either cyclically or constitutively expressed Bmal1 was able to restore circadian rhythmicity in Bmal1−/− cells. We suggest that the robustness of the core loop in the absence of rhythmic BMAL1 is retained by coordinated regulation of transcriptional and post-translational mechanisms, including particularly protein turnover and synchronous nuclear translocation of PER/CRY proteins despite the differential phases and/or lack of rhythmicity of their mRNAs (see discussion). In both the core loop and ROR/REV/Bmal1 loop, the repressors play more dominant roles than the activators. (B) The interlocking loops connect the core loop to temporal regulation of local output networks. Peripheral tissues are coordinated by the SCN in vivo, and the states of peripheral oscillators are also influenced by behavior, physiology, and pathology. The core loop directly controls expression of 1st order CCGs, subsequently forming a cascade of rhythmic gene expression. The net result of this cascade is the appropriately timed production of proteins important for local physiology, which collectively contribute to coordinated circadian behavior and physiology at the organismal level. In this context, the interlocking loops, including the ROR/REV/Bmal1 loop and its constituents, are 1st order CCGs and serve as important transmitters or integrators for local circadian biology.
Similar articles
- The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator.
Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. Preitner N, et al. Cell. 2002 Jul 26;110(2):251-60. doi: 10.1016/s0092-8674(02)00825-5. Cell. 2002. PMID: 12150932 - Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors.
Guillaumond F, Dardente H, Giguère V, Cermakian N. Guillaumond F, et al. J Biol Rhythms. 2005 Oct;20(5):391-403. doi: 10.1177/0748730405277232. J Biol Rhythms. 2005. PMID: 16267379 - REV-ERBα and REV-ERBβ function as key factors regulating Mammalian Circadian Output.
Ikeda R, Tsuchiya Y, Koike N, Umemura Y, Inokawa H, Ono R, Inoue M, Sasawaki Y, Grieten T, Okubo N, Ikoma K, Fujiwara H, Kubo T, Yagita K. Ikeda R, et al. Sci Rep. 2019 Jul 15;9(1):10171. doi: 10.1038/s41598-019-46656-0. Sci Rep. 2019. PMID: 31308426 Free PMC article. - Genetics and neurobiology of circadian clocks in mammals.
Siepka SM, Yoo SH, Park J, Lee C, Takahashi JS. Siepka SM, et al. Cold Spring Harb Symp Quant Biol. 2007;72:251-259. doi: 10.1101/sqb.2007.72.052. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419282 Free PMC article. Review. - Circadian stabilization loop: the regulatory hub and therapeutic target promoting circadian resilience and physiological health.
Kim E, Yoo SH, Chen Z. Kim E, et al. F1000Res. 2022 Oct 31;11:1236. doi: 10.12688/f1000research.126364.2. eCollection 2022. F1000Res. 2022. PMID: 36415204 Free PMC article. Review.
Cited by
- Circadian Amplitude Regulation via FBXW7-Targeted REV-ERBα Degradation.
Zhao X, Hirota T, Han X, Cho H, Chong LW, Lamia K, Liu S, Atkins AR, Banayo E, Liddle C, Yu RT, Yates JR 3rd, Kay SA, Downes M, Evans RM. Zhao X, et al. Cell. 2016 Jun 16;165(7):1644-1657. doi: 10.1016/j.cell.2016.05.012. Epub 2016 May 26. Cell. 2016. PMID: 27238018 Free PMC article. - Autonomous and self-sustained circadian oscillators displayed in human islet cells.
Pulimeno P, Mannic T, Sage D, Giovannoni L, Salmon P, Lemeille S, Giry-Laterriere M, Unser M, Bosco D, Bauer C, Morf J, Halban P, Philippe J, Dibner C. Pulimeno P, et al. Diabetologia. 2013 Mar;56(3):497-507. doi: 10.1007/s00125-012-2779-7. Epub 2012 Dec 15. Diabetologia. 2013. PMID: 23242133 Free PMC article. - Cryptochrome 1 regulates the circadian clock through dynamic interactions with the BMAL1 C terminus.
Xu H, Gustafson CL, Sammons PJ, Khan SK, Parsley NC, Ramanathan C, Lee HW, Liu AC, Partch CL. Xu H, et al. Nat Struct Mol Biol. 2015 Jun;22(6):476-484. doi: 10.1038/nsmb.3018. Epub 2015 May 11. Nat Struct Mol Biol. 2015. PMID: 25961797 Free PMC article. - Crosstalk of clock gene expression and autophagy in aging.
Kalfalah F, Janke L, Schiavi A, Tigges J, Ix A, Ventura N, Boege F, Reinke H. Kalfalah F, et al. Aging (Albany NY). 2016 Aug 28;8(9):1876-1895. doi: 10.18632/aging.101018. Aging (Albany NY). 2016. PMID: 27574892 Free PMC article. - Circadian regulation of MGMT expression and promoter methylation underlies daily rhythms in TMZ sensitivity in glioblastoma.
Gonzalez-Aponte MF, Damato AR, Trebucq LL, Simon T, Cárdenas-García SP, Cho K, Patti GJ, Golombek DA, Chiesa JJ, Rubin JB, Herzog ED. Gonzalez-Aponte MF, et al. J Neurooncol. 2024 Feb;166(3):419-430. doi: 10.1007/s11060-023-04535-9. Epub 2024 Jan 26. J Neurooncol. 2024. PMID: 38277015 Free PMC article.
References
- Young MW, Kay SA. Time zones: A comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. - PubMed
- Liu AC, Lewis WG, Kay SA. Mammalian circadian signaling networks and therapeutic targets. Nat Chem Biol. 2007;3:631–639. - PubMed
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. - PubMed
- Giguere V. Orphan nuclear receptors: From gene to function. Endocrine Rev. 1999;20:689–725. - PubMed
- Preitner N, Damiola F, Molina LL, Zakany J, Duboule D, et al. The orphan nuclear receptor REV-ERB alpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases