A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation - PubMed (original) (raw)
. 2008 May 2;133(3):462-74.
doi: 10.1016/j.cell.2008.02.048.
Mei-ling A Joiner, Xiaoqun Guan, William Kutschke, Jinying Yang, Carmine V Oddis, Ryan K Bartlett, John S Lowe, Susan E O'Donnell, Nukhet Aykin-Burns, Matthew C Zimmerman, Kathy Zimmerman, Amy-Joan L Ham, Robert M Weiss, Douglas R Spitz, Madeline A Shea, Roger J Colbran, Peter J Mohler, Mark E Anderson
Affiliations
- PMID: 18455987
- PMCID: PMC2435269
- DOI: 10.1016/j.cell.2008.02.048
A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation
Jeffrey R Erickson et al. Cell. 2008.
Abstract
Calcium/calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII) couples increases in cellular Ca2+ to fundamental responses in excitable cells. CaMKII was identified over 20 years ago by activation dependence on Ca2+/CaM, but recent evidence shows that CaMKII activity is also enhanced by pro-oxidant conditions. Here we show that oxidation of paired regulatory domain methionine residues sustains CaMKII activity in the absence of Ca2+/CaM. CaMKII is activated by angiotensin II (AngII)-induced oxidation, leading to apoptosis in cardiomyocytes both in vitro and in vivo. CaMKII oxidation is reversed by methionine sulfoxide reductase A (MsrA), and MsrA-/- mice show exaggerated CaMKII oxidation and myocardial apoptosis, impaired cardiac function, and increased mortality after myocardial infarction. Our data demonstrate a dynamic mechanism for CaMKII activation by oxidation and highlight the critical importance of oxidation-dependent CaMKII activation to AngII and ischemic myocardial apoptosis.
Figures
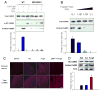
Figure 1. CaMKII is activated by ROS
(A) General structure of a subunit from the multimeric holoenzyme CaMKII and mechanism of CaMKII activation by autophosphorylation. The amino acid sequence of the regulatory domain is highlighted to show the autoinhibitory (AI) and calmodulin-binding (CaM-B) regions. Yellow symbols represent CaM. Pretreatment with Ca2+/CaM (1°) followed by phosphorylation at T287 (2°) yields persistent activity even after the removal of Ca2+/CaM (3°). (B) Kinase assays were performed after three distinct treatment steps: (1°) ± Ca2+/CaM, (2°) ± H2O2 or ATP, and (3°) ± EGTA. (n = 6 assays/group, * p < 0.05 vs. WT no treatment). (C) CaMKII is activated by H2O2 in a dose-dependent manner after pre-treatment with Ca2+/CaM. Oxidation-dependent CaMKII activity is ablated in M281/282V mutants (n = 6 assays/group, * p < 0.05 vs. WT no treatment). (D) M281/282V mutants have normal Ca2+/CaM-dependent and T287-autophosphorylation-dependent activation (n = 6 assays/group, * p < 0.05 vs. WT no treatment). (E) Proposed mechanism for activation of CaMKII by oxidation. After initial activation of the holoenzyme by Ca2+/CaM (1°), oxidation at M281/282 (2°) blocks reassociation of the catalytic domain, yielding persistent CaMKII activity (3°).
Figure 2. AngII induces oxidation of CaMKII in vivo
(A) Immunoblot of WT CaMKII and M281/282V mutant after no treatment, oxidation, or autophosphorylation probed with antibodies against total, autophosphorylated (p-T287), or oxidized CaMKII. Summary data shows relative band intensity using the oxidized CaMKII antibody (n = 3 trials/group, * p < 0.05 vs. band intensity of WT CaMKII treated with H2O2). (B) Immunoblot and summary data of oxidized WT CaMKII probed with antiserum against oxidized M281/282 with increasing ratios of oxidized antigen peptide. (n = 3 trials/group, * p < 0.05 vs. band intensity with no peptide). (C) Immunofluorescent staining of heart sections from mice treated with saline, AngII, or Iso and probed for oxidized or total CaMKII. Red staining is positive for oxidized or total CaMKII and blue staining is for nuclei. Calibration bars are 100 microns. (D) Immunoblot and summary data of heart lysates from mice treated with saline (Sal), Iso, or AngII probed with antibodies against total CaMKII, oxidized CaMKII, or actin (n = 3 hearts/group, * p < 0.05 vs. band intensity of saline treatment).
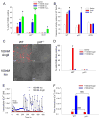
Figure 3. AngII increases ROS production and apoptosis by a CaMKII-dependent pathway in cardiomyocytes
(A) Percent of total isolated cardiomyocytes positive for TUNEL staining after treatment with saline, AngII, Iso, or H2O2 (n = 6 hearts/group, * p < 0.05 vs. WT with saline). (B) Caspase-3 activity induced by saline, AngII, or Iso normalized to WT cells treated with saline (n = 3 hearts/group, * p < 0.05 vs. WT with saline). (C) DHE stained cardiomyocytes after treatment with 100nM AngII or Iso. Red coloration indicates presence of ROS above control cells. Scale bars equal 50 μm. (D) Percent of total cells positive for DHE staining above control (n = 3 assays/group, * p < 0.05 vs. WT saline). (E) Example traces of intracellular calcium concentration of cultured WT cardiomyocytes treated with 100nM AngII (red symbols) or Iso (blue symbols) measured by real-time calcium imaging. The arrow indicates addition of AngII or Iso. (F) Peak intracellular Ca2+ concentration in response to either AngII or Iso for WT or p47−/− cells (n = 3 trials/group, NS = not statistically different).
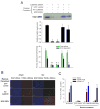
Figure 4. AngII-induced apoptosis is blocked by CaMKII silencing
(A) Representative immunoblot with anti-CaMKII to measure protein expression after treatment with shRNA and shRNA-resistant rescue constructs. Immunoblot against actin was used as a loading control (not shown). Middle panel shows summary data of CaMKII expression relative to untreated cells (n = 3 experiments/group, * p < 0.05 vs. no treatment). Bottom panel shows summary data for CaMKII activity assays of lysates (n = 3 experiments/group, * p < 0.05 vs. total activity with no treatment, † p < 0.05 vs. ROS-dependent activity with no treatment). Only the WT CaMKII construct was able to reconstitute both Ca2+/CaM- and ROS-dependent activity observed in untreated cells. (B) Immunostaining and (C) summary data from isolated rat cardiomyocytes transduced with shRNA against CaMKII followed by rescue with WT CaMKII, M281/282V, or GFP control. Immunostaining shows total nuclei (DAPI) and DNA nicking (TUNEL) consistent with apoptosis. Scale bars equal 100 μm. Summary data show percent of total nuclei with positive TUNEL staining (n = 6 hearts/group, * p < 0.05 vs. GFP with AngII).
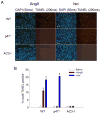
Figure 5. AngII causes cardiac apoptosis in vivo via a ROS and CaMKII-mediated pathway
(A) Immunostaining of mouse heart sections for total nuclei (DAPI) and nuclear damage (TUNEL) consistent with apoptosis. WT, p47−/−, and AC3-I mice were treated with Ang II (3mg/kg/day) or Iso (30mg/kg/day) for seven days. Scale bars equal 100 μm. (B) Percent of total nuclei that showed positive TUNEL staining (n = 3 hearts/group, * p < 0.05 vs. WT with saline).
Figure 6. MsrA−/− mice have increased susceptibility to AngII-mediated apoptosis
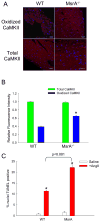
(A) Immunofluorescent staining of heart sections from WT and MsrA−/− mice treated with AngII and probed for oxidized or total CaMKII. Red staining is positive for oxidized or total CaMKII and blue staining is for nuclei. Calibration bars are 100 microns. (B) Quantification of average staining intensity for AngII treated hearts, relative to WT (n = 3 hearts/group, * p < 0.05 vs. WT with AngII). (C) Summary data for TUNEL staining of heart sections from WT and MsrA−/− mice treated with saline or AngII (n = 5 hearts/group, * p < 0.05 vs. WT with saline).
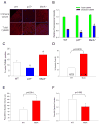
Figure 7. Mice lacking MsrA have increased CaMKII oxidation, apoptosis, reduced survival and impaired heart function after myocardial infarction
(A) Immunostaining and (B) stain intensity quantification of oxidized CaMKII in heart sections from WT, p47−/−, and MsrA−/− mice post-MI (n = 3 hearts/group, * p < 0.05 vs. WT). (C) Summary data for TUNEL staining of heart sections from WT, p47−/−, and MsrA−/− mice post-MI (n = 3 hearts/group, * p < 0.05 vs. WT). (D) Mortality is significantly increased post-MI in MsrA−/− mice compared to WT controls. Numbers in bars represent post-MI deaths/total number of mice receiving MI. Post-MI left ventricular dilation (E) and function (F) were compromised in surviving MsrA−/− mice compared to WT controls three weeks after surgery (n = 17 hearts/group for WT, n = 9 hearts/group for MsrA−/−).
Comment in
- CaMKII: new tricks for an old dog.
Griffith LC. Griffith LC. Cell. 2008 May 2;133(3):397-9. doi: 10.1016/j.cell.2008.04.018. Cell. 2008. PMID: 18455979 Free PMC article.
Similar articles
- Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species.
Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Palomeque J, et al. Circ Res. 2009 Dec 4;105(12):1204-12. doi: 10.1161/CIRCRESAHA.109.204172. Epub 2009 Oct 22. Circ Res. 2009. PMID: 19850941 - Novel Roles for Peroxynitrite in Angiotensin II and CaMKII Signaling.
Zhou C, Ramaswamy SS, Johnson DE, Vitturi DA, Schopfer FJ, Freeman BA, Hudmon A, Levitan ES. Zhou C, et al. Sci Rep. 2016 Apr 15;6:23416. doi: 10.1038/srep23416. Sci Rep. 2016. PMID: 27079272 Free PMC article. - Site-specific methionine oxidation in calmodulin affects structural integrity and interaction with Ca2+/calmodulin-dependent protein kinase II.
Snijder J, Rose RJ, Raijmakers R, Heck AJ. Snijder J, et al. J Struct Biol. 2011 Apr;174(1):187-95. doi: 10.1016/j.jsb.2010.12.002. Epub 2010 Dec 13. J Struct Biol. 2011. PMID: 21156208 - CaMKII oxidative activation and the pathogenesis of cardiac disease.
Luczak ED, Anderson ME. Luczak ED, et al. J Mol Cell Cardiol. 2014 Aug;73:112-6. doi: 10.1016/j.yjmcc.2014.02.004. Epub 2014 Feb 13. J Mol Cell Cardiol. 2014. PMID: 24530899 Free PMC article. Review. - CaMKII is a nodal signal for multiple programmed cell death pathways in heart.
Feng N, Anderson ME. Feng N, et al. J Mol Cell Cardiol. 2017 Feb;103:102-109. doi: 10.1016/j.yjmcc.2016.12.007. Epub 2016 Dec 24. J Mol Cell Cardiol. 2017. PMID: 28025046 Free PMC article. Review.
Cited by
- PKM2 regulates metabolic flux and oxidative stress in the murine heart.
Lee KCY, Williams AL, Wang L, Xie G, Jia W, Fujimoto A, Gerschenson M, Shohet RV. Lee KCY, et al. Physiol Rep. 2024 Sep;12(17):e70040. doi: 10.14814/phy2.70040. Physiol Rep. 2024. PMID: 39256891 Free PMC article. - The Role of Sodium Fluoride Mouthwash in Regulating FGF-2 and TGF-β Expression in Human Gingival Fibroblasts.
Kato N, Nakai K, Tanaka H, Fukuzawa K, Hayashi M, Aoki M, Kawato T. Kato N, et al. Biomedicines. 2024 Aug 1;12(8):1727. doi: 10.3390/biomedicines12081727. Biomedicines. 2024. PMID: 39200192 Free PMC article. - Mitochondrial calcium signaling and redox homeostasis in cardiac health and disease.
Popoiu TA, Maack C, Bertero E. Popoiu TA, et al. Front Mol Med. 2023 Aug 23;3:1235188. doi: 10.3389/fmmed.2023.1235188. eCollection 2023. Front Mol Med. 2023. PMID: 39086688 Free PMC article. Review. - The inotropic and arrhythmogenic effects of acutely increased late INa are associated with elevated ROS but not oxidation of PKARIα.
Gissibl T, Stengel L, Tarnowski D, Maier LS, Wagner S, Feder AL, Sag CM. Gissibl T, et al. Front Cardiovasc Med. 2024 Jul 15;11:1379930. doi: 10.3389/fcvm.2024.1379930. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39077112 Free PMC article. - Heart rate-corrected systolic ejection time: population-based reference values and differential prognostic utility in acute heart failure.
Morbach C, Simon I, Danner E, Gelbrich G, Stefenelli U, Sahiti F, Scholz N, Cejka V, Albert J, Ertl G, Angermann CE, Güder G, Frantz S, Heuschmann PU, Maack C, Störk S. Morbach C, et al. Eur Heart J Imaging Methods Pract. 2023 Sep 12;1(2):qyad020. doi: 10.1093/ehjimp/qyad020. eCollection 2023 Sep. Eur Heart J Imaging Methods Pract. 2023. PMID: 39045077 Free PMC article.
References
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. - PubMed
- Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol. 2004;14:679–686. - PubMed
- Bers DM. Beyond beta blockers. Nat Med. 2005;11:379–380. - PubMed
- Colbran RJ. Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem. 1993;268:7163–7170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH063232-08/MH/NIMH NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- R01 HL070250-06A1/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- R01 HL079031-02/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- RR017369/RR/NCRR NIH HHS/United States
- R01 MH063232/MH/NIMH NIH HHS/United States
- R01 HL062494-09/HL/NHLBI NIH HHS/United States
- R01 GM057001/GM/NIGMS NIH HHS/United States
- R01 HL 62494/HL/NHLBI NIH HHS/United States
- K26 RR017369/RR/NCRR NIH HHS/United States
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R01 HL 079031/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- R01 GM57001/GM/NIGMS NIH HHS/United States
- R01 HL 70250/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous