Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1 - PubMed (original) (raw)
Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1
Andrew Y Kim et al. Cell. 2008.
Abstract
Transient receptor potential vanilloid 1 (TRPV1) is a molecular sensor of noxious heat and capsaicin. Its channel activity can be modulated by several mechanisms. Here we identify a membrane protein, Pirt, as a regulator of TRPV1. Pirt is expressed in most nociceptive neurons in the dorsal root ganglia (DRG) including TRPV1-positive cells. Pirt null mice show impaired responsiveness to noxious heat and capsaicin. Noxious heat- and capsaicin-sensitive currents in Pirt-deficient DRG neurons are significantly attenuated. Heterologous expression of Pirt strongly enhances TRPV1-mediated currents. Furthermore, the C terminus of Pirt binds to TRPV1 and several phosphoinositides, including phosphatidylinositol-4,5-bisphosphate (PIP2), and can potentiate TRPV1. The PIP2 binding is dependent on the cluster of basic residues in the Pirt C terminus and is crucial for Pirt regulation of TRPV1. Importantly, the enhancement of TRPV1 by PIP2 requires Pirt. Therefore, Pirt is a key component of the TRPV1 complex and positively regulates TRPV1 activity.
Figures
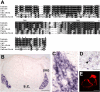
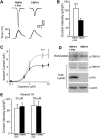
Figure 1. Pirt Encodes a Two Transmembrane Domain Protein and Is Expressed Predominantly in DRG
(A) Alignment of amino acid sequences of Pirt from various vertebrates. Residues shaded in black are identical in >50% of the predicted proteins; similar residues are highlighted in gray. The predicted transmembrane domains (TM1 and TM2) and positively charged residues in the C-terminal region (asterisk) are indicated. (B–D) In situ hybridization with cRNA probes detecting Pirt. (B) Cross-section through the trunk region of E14.5 mouse embryo. Strong Pirt signal was present in DRG but absent in the spinal cord (s.c.). (C) A higher magnification view of the DRG region. (D) Cross-section of sympathetic ganglia from E14.5 embryo. A small subset of neurons was stained (arrowhead). (E) Membrane localization of Pirt. Pirt cDNA was tagged with a myc epitope at the C terminus of the protein and expressed in PC12 cells. When the plasma membrane was permeabilized by detergent, membrane localization of the Pirt-myc fusion protein was observed by anti-myc antibody staining (two adjacent cells shown). Under nondetergent conditions no specific staining was found (data not shown). Similar results were obtained using an antibody against the N-terminus of Pirt (data not shown). Scale bars in (B), (C), (D), and (E) represent 100, 50, 40, and 10 μm, respectively.
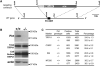
Figure 2. Generation of Pirt Null Mice
(A) Targeting strategy for disrupting the Pirt gene. The entire ORF of Pirt (black box) was replaced by farnesylated enhanced GFP (EGFPf) followed by IRES-reverse-tetracycline-transactivator (IRES-rtTA). ACN is a self-excising neomycin expression cassette. (B) Western blots of DRG lysates derived from Pirt+/+ and _Pirt_−/− mice using antibodies specifically against Pirt, TRPV1, and β-actin demonstrate that Pirt protein is specifically deleted in _Pirt_−/− mice. TRPV1 expression in cultured DRG neurons was measured by western blot after plasma membrane proteins were biotinylated and purified with streptavidin. The molecular weights are indicated on the right. (C) Immunofluorescence staining of DRG sections show normal proportions of TRPV1-, CGRP-, IB4-, and NF200-expressing neurons in _Pirt_−/− mice (n = 3). The total number of DRG neurons was determined by costaining with the pan-neuronal marker NeuN (Lo et al., 2002). Values are mean ± standard error.
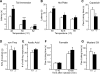
Figure 3. _Pirt_−/− Mice Show Impaired Behavioral Responses to Noxious Heat and Capsaicin
(A) Tail-withdrawal latency of _Pirt_−/− mice (black bars) in the tail immersion test was significantly increased as compared with Pirt+/+ mice (white bars) when water bath temperature was at 48°C (Pirt+/+: n = 15; _Pirt_−/−: n = 16), 50°C (+/+: n = 21; −/−: n = 21), and 52°C (+/+: n = 9; −/−: n = 10). (B) Paw-withdrawal latency of _Pirt_−/− mice on hot plate was significantly longer than in WT mice at 5C (+/+: n = 21; −/−: n = 22) and 52.5°C (+/+: n = 15; −/−: n = 17) but not at 55°C (+/+: n = 11; −/−: n = 14). (C) Behavioral responses to intraplantar injection of capsaicin (0.6 μg) were measured for Pirt+/+ (n = 18) and _Pirt_−/− (n = 18) mice for 10 min after injection. (D) Responsiveness to mechanical stimulation as measured by von Frey test was the same in WT (n = 8) and mutant (n = 9) mice. (E) _Pirt_−/− mice (n = 18) showed a similar number of writhes in response to intraperitoneal acetic acid injection compared with WT mice (n = 16). (F) Paw licking times during phase I (0−10 min) and phase II (10−60 min) of the nociceptive response to formalin injection (Tjolsen et al., 1992) were not significantly different between the two genotypes (n = 14/genotype). (G) Behavioral responses to intraplantar injection of mustard oil (6 μl of 2.0%) were measured for Pirt+/+ and _Pirt_−/− mice (n = 13/genotype) for 10 min after injection. *p < 0.05; **p < 0.01; ***p < 0.001 for Pirt+/+ versus _Pirt_−/−; two-tailed unpaired t test. All error bars represent standard error of the mean (SEM).
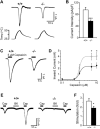
Figure 4. Heat- and Capsaicin-Evoked Currents Were Reduced in Pirt-Deficient DRG Neurons
(A) Responses of DRG neurons from Pirt+/+ (+/+) and _Pirt_−/− (−/−) mice to heat. Current trace and temperature ramp (25°C –50°C) are shown on the top and bottom, respectively. (B) Current intensities of DRG neuron response to heat. Current intensities of WT (open bar, n = 39) and _Pirt_−/− (black bar, n = 33) neurons were significantly different (***p < 0.005). (C) Inward current response to application of 5 μM capsaicin (black line) in DRG neurons from Pirt+/+ (+/+) and _Pirt_−/− (−/−) mice. (D) The concentration-response relationship expressed as inward current versus concentration of capsaicin in WT (open circles) and _Pirt_−/− (closed circles) neurons. Data points were fitted to the Hill equation. When 5 μM and 10 μM capsaicin were applied to DRG culture neurons of WT (n = 26 in 5 μM, n = 25 in 10 μM) and _Pirt_−/− (n = 26 at 5 μM, n = 20 at 10 μM) mice, respectively, the current sizes (*p < 0.05) and current intensities (data not shown) were significantly different. (E) Representative current traces from Pirt+/+ (n = 15) and _Pirt_−/− (n = 16) neurons stimulated with capsaicin (0.1 μM, black line) followed by bradykinin (1 μM, gray line) and capsaicin (0.1 μM) again. (F) Potentiation by bradykinin was calculated by dividing post-treatment capsaicin-evoked currents by pre-treatment values. Wild-type neurons showed stronger potentiation by bradykinin than _Pirt_−/− neurons (p = 0.041). All error bars represent SEM.
Figure 5. TRPV1-Mediated Currents Are Enhanced by Expression of Pirt
(A) Heat-evoked current traces in TRPV1 stably expressing HEK293 cells. Top shows current traces in TRPV1 cells cotransfected with Pirt and GFP (TRPV1 + Pirt) and TRPV1 cells transfected with GFP alone (TRPV1); bottom shows the ramp of heated buffer application. (B) Heat-evoked current intensities of TRPV1 cells expressing Pirt (open bar, n = 30) or TRPV1 alone (black bar, n = 30). Pirt significantly increases TRPV1 current in response to heat stimulation (***p < 0.005). (C) Capsaicin dose-response curves of TRPV1 stably expressing cells cotransfected with Pirt and GFP (open circles) and TRPV1 cells transfected with GFP alone (closed circles). When 5 μM and 10 μM capsaicin were applied to TRPV1/Pirt cells (n = 26 at 5 μM, n = 16 at 10 μM) and TRPV1 cells (n = 26 at 5 μM, n = 15 at 10 μM), respectively, the current sizes and current intensities (data not shown) indicated significantly different responses. Holding potential = −60 mV. (D) The expression level of TRPV1 in the TRPV1 stable HEK293 cells was measured by western blot after plasma membrane proteins were biotinylated and purified with streptavidin. β-actin was used as a loading control. (E) Mustard oil-evoked current intensities in TRPA1 stably expressing HEK293 cells. Twenty and one hundred micromolars of mustard oil were used to stimulate the cells. The current intensities of TRPA1 cells expressing Pirt (open bar, n = 12) and TRPA1 alone (black bar, n = 12) were almost identical at both concentrations. All error bars represent SEM.
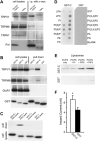
Figure 6. Pirt Binds to TRPV1 and PIPs
(A) Coimmunoprecipitation of TRP channels and Pirt in HEK293 cells. HEK293 cells transfected with different TRPs and Pirt-myc were immunoprecipitated with anti-myc antibody. Immunoprecipitates were detected by antibodies against corresponding TRPs and Pirt. The immunoblots of TRPs and Pirt in cell lysates indicate their expression levels before immunoprecipitation. Pirt forms a complex with TRPV1 and TRPM8 but not TRPA1. (B) GST pull-down assay of HEK293 cell lysates coexpressing TRPV1 with GST-N, GST-C, or GST alone and visualized by western blot analysis with anti-TRPV1 antibody. GST pull down of HEK293 cell lysates coexpressing either TRPM8 or HA-tagged GluR1 with GST-N, GST-C, and GST alone and visualized by western blot analysis with anti-TRPM8 or anti-HA antibodies, respectively. (C) GST-C binds PIP2-conjugated agarose beads. PIP2-conjugated agarose beads were used to pull down GST-N, GST-C, and GST from HEK293 cell lysates expressing these proteins. Control beads without PIP2 were also used to pull down GST-C. (D) Protein-lipid overlays. Cell lysates from GST-C fusion protein comprised of the Pirt C terminus (left panel) or GST only (right panel) transfected HEK293 cells were used to probe PIP strips. For each strip, lipids were deposited in two rows and only labeled outside. The proteins were detected by ECL after probing with anti-GST antibodies. Strong binding of GST-C to PIPs, PIP2, and PIP3 was observed but no binding was found with control lipids such as phosphatidylinositol (PI) and phosphatidylethanolamine (PE). (E) Liposomal pulldown assay. Liposomes composed of phosphatidylcholine (PC), phosphatidylserine (PS), and different PIPs were prepared as indicated in Experimental Procedures. PI-, PIP2-, or PIP3-containing or control (composed of PC and PS only) liposomes were incubated with the same amount (0.1 μg) of purified GST-C or GST and pelleted by centrifugation. Associated proteins were visualized by western blot with anti-GST antibody. (F) Heat-evoked current sizes of TRPV1 cells expressing GST-C (open bar, n = 29) or GST alone (black bar, n = 33). The C terminus of Pirt can significantly increase TRPV1 current response to heat stimulation (*p < 0.05). All error bars represent SEM.
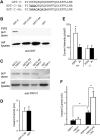
Figure 7. Pirt and PIP2 Are Dependent on Each Other to Enhance TRPV1 Activity
(A) Sequences of WT and mutant forms of the Pirt C terminus, which were fused with GST. Bold letters indicate the mutated basic residues. (B) PIP2-conjugated agarose beads failed to pull down GST-C-3Q and GST-C-6A from HEK293 cell lysates expressing these proteins. The pull-down products were immunoblotted by antibody against GST. (C) Binding of TRPV1 with GST-C-3Q or GST-C-6A in HEK293 cells. HEK293 cells transfected with TRPV1 and either GST-C, GST-C-3Q, or GST-C-6A were pulled down with glutathione-agarose beads. Immunoprecipitates were immunoblotted by anti-TRPV1 antibody. The mutant C termini still formed a complex with TRPV1. (D) Heat-evoked current sizes of TRPV1 stably expressing cells expressing GST-C-3Q (open bar, n = 18) or GST alone (black bar, n = 18). The mutant C terminus has no effect on TRPV1 activity. (E) Current densities of DRG neurons from Pirt+/+ (+/+) and _Pirt_−/− (−/−) mice in response to 5 μM capsaicin. Whole-cell recordings were performed with either water-soluble PIP2-diC8 (10 μM; Echelon Biosciences; open bars) or PI-diC8 (10 μM; black bar) added to the pipette solution. In WT neurons, PIP2 (n = 27) significantly enhanced TRPV1-mediated current as compared to PI (n = 25; p = 0.017). In _Pirt_−/− neurons, PIP2 (n = 25) did not show any effect on TRPV1 current as compared to PI (n = 19). (F) Currents of HEK293 cells transfected with WT TRPV1 or TRPV1Δ42 in the presence or absence of Pirt in response to 0.1 μM capsaicin. Cells expressing TRPV1Δ42 alone (n = 9) showed significantly stronger current than those expressing WT channels (n = 11). Coexpressing Pirt can significantly potentiate TRPV1Δ42- (n = 10) but not WT TRPV1- (n = 12) mediated currents (*p < 0.05). All error bars represent SEM.
Similar articles
- Localization of the PIP2 sensor of TRPV1 ion channels.
Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. Ufret-Vincenty CA, et al. J Biol Chem. 2011 Mar 18;286(11):9688-98. doi: 10.1074/jbc.M110.192526. Epub 2011 Jan 11. J Biol Chem. 2011. PMID: 21224382 Free PMC article. - Pirt Together with TRPV1 Is Involved in the Regulation of Neuropathic Pain.
Wang C, Gu L, Ruan Y, Gegen T, Yu L, Zhu C, Yang Y, Zhou Y, Yu G, Tang Z. Wang C, et al. Neural Plast. 2018 Apr 2;2018:4861491. doi: 10.1155/2018/4861491. eCollection 2018. Neural Plast. 2018. PMID: 29808083 Free PMC article. - Dual regulation of TRPV1 by phosphoinositides.
Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Lukacs V, et al. J Neurosci. 2007 Jun 27;27(26):7070-80. doi: 10.1523/JNEUROSCI.1866-07.2007. J Neurosci. 2007. PMID: 17596456 Free PMC article. - Is thermal nociception only sensed by the capsaicin receptor, TRPV1?
Hiura A. Hiura A. Anat Sci Int. 2009 Sep;84(3):122-8. doi: 10.1007/s12565-009-0048-8. Epub 2009 Jun 27. Anat Sci Int. 2009. PMID: 19562439 Review. - Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review.
Cited by
- Integrative binding sites within intracellular termini of TRPV1 receptor.
Grycova L, Holendova B, Bumba L, Bily J, Jirku M, Lansky Z, Teisinger J. Grycova L, et al. PLoS One. 2012;7(10):e48437. doi: 10.1371/journal.pone.0048437. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119017 Free PMC article. - Construction of a global pain systems network highlights phospholipid signaling as a regulator of heat nociception.
Neely GG, Rao S, Costigan M, Mair N, Racz I, Milinkeviciute G, Meixner A, Nayanala S, Griffin RS, Belfer I, Dai F, Smith S, Diatchenko L, Marengo S, Haubner BJ, Novatchkova M, Gibson D, Maixner W, Pospisilik JA, Hirsch E, Whishaw IQ, Zimmer A, Gupta V, Sasaki J, Kanaho Y, Sasaki T, Kress M, Woolf CJ, Penninger JM. Neely GG, et al. PLoS Genet. 2012;8(12):e1003071. doi: 10.1371/journal.pgen.1003071. Epub 2012 Dec 6. PLoS Genet. 2012. PMID: 23236288 Free PMC article. - Phosphoinositides: tiny lipids with giant impact on cell regulation.
Balla T. Balla T. Physiol Rev. 2013 Jul;93(3):1019-137. doi: 10.1152/physrev.00028.2012. Physiol Rev. 2013. PMID: 23899561 Free PMC article. Review. - Co-localization of Pirt protein and P2X2 receptors in the mouse enteric nervous system.
Guo W, Sui QQ, Gao XF, Feng JF, Zhu J, He C, Knight GE, Burnstock G, Xiang Z. Guo W, et al. Purinergic Signal. 2016 Sep;12(3):489-96. doi: 10.1007/s11302-016-9515-6. Epub 2016 Apr 22. Purinergic Signal. 2016. PMID: 27105971 Free PMC article. - Exploring neuronal mechanisms involved in the scratching behavior of a mouse model of allergic contact dermatitis by transcriptomics.
Liu B, Chen R, Wang J, Li Y, Yin C, Tai Y, Nie H, Zeng D, Fang J, Du J, Liang Y, Shao X, Fang J, Liu B. Liu B, et al. Cell Mol Biol Lett. 2022 Feb 19;27(1):16. doi: 10.1186/s11658-022-00316-w. Cell Mol Biol Lett. 2022. PMID: 35183104 Free PMC article.
References
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
- Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr. Opin. Neurobiol. 1999;9:525–530. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS054791-01A1/NS/NINDS NIH HHS/United States
- T32 EY017203/EY/NEI NIH HHS/United States
- R37 NS054791/NS/NINDS NIH HHS/United States
- NS054791/NS/NINDS NIH HHS/United States
- R01 NS054791-03/NS/NINDS NIH HHS/United States
- R01 NS054791/NS/NINDS NIH HHS/United States
- R01 NS054791-04/NS/NINDS NIH HHS/United States
- R01 NS054791-02/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases