Kindlin-2 (Mig-2): a co-activator of beta3 integrins - PubMed (original) (raw)
Kindlin-2 (Mig-2): a co-activator of beta3 integrins
Yan-Qing Ma et al. J Cell Biol. 2008.
Abstract
Integrin activation is essential for dynamically linking the extracellular environment and cytoskeletal/signaling networks. Activation is controlled by integrins' short cytoplasmic tails (CTs). It is widely accepted that the head domain of talin (talin-H) can mediate integrin activation by binding to two sites in integrin beta's CT; in integrin beta(3) this is an NPLY(747) motif and the membrane-proximal region. Here, we show that the C-terminal region of integrin beta(3) CT, composed of a conserved TS(752)T region and NITY(759) motif, supports integrin activation by binding to a cytosolic binding partner, kindlin-2, a widely distributed PTB domain protein. Co-transfection of kindlin-2 with talin-H results in a synergistic enhancement of integrin alpha(IIb)beta(3) activation. Furthermore, siRNA knockdown of endogenous kindlin-2 impairs talin-induced alpha(IIb)beta(3) activation in transfected CHO cells and blunts alpha(v)beta(3)-mediated adhesion and migration of endothelial cells. Our results thus identify kindlin-2 as a novel regulator of integrin activation; it functions as a coactivator.
Figures
Figure 1.
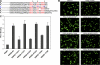
Sequences of the membrane-distal region of β3 CT have essential roles in integrin αIIbβ3 activation. (A) Alignment of integrin β CT sequences, highlighting (red) the conserved regions, the two NXXY/F motifs and one T/S cluster. (B) Suppression of integrin αIIbβ3-mediated cell spreading by expressed β3 CT depends on conserved sequences in its membrane-distal region. After transient transfection with plasmids encoding the indicated β3 CT-containing chimera (β3 CT/PSGL-1), adhesion of the αIIbβ3-CHO cells to fibrinogen was examined. The adherent cells were fixed and stained with the anti-PSGL-1 mAb, KPL-1, for visualization by fluorescence microscopy (10× objective). Bar, 20 μm. (C) Conserved residues in the membrane-distal region support αIIbβ3 activation. Plasmids encoding αIIb and β3 or its mutants were transiently transfected to CHO cells. The transfected cells were stained with 2G12 to assess αIIbβ3 expression or PAC1 to assess αIIbβ3 activation. FACS was used to measure the mean fluorescence intensity (MFI) of 2G12 or PAC1 binding, and relative MFI of PAC1 binding was normalized to integrin expression levels based on 2G12 staining (Ma et al., 2006). The error bars represent means ± SD of three independent experiments.
Figure 2.
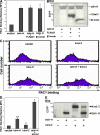
Kindlin-2 enhances talin-induced integrin αIIbβ3 activation. EGFP-fused β3 CT binding proteins were transiently transfected into αIIbβ3-CHO cells. Their effects on αIIbβ3 activation were evaluated by PAC1 binding (A and D) and expression levels were measured by Western blotting with anti-GFP antibody (B and E). Representative FACS histograms of PAC1 binding to talin-H and/or kindin-2 (kind-2) positive cells (C). Error bars (A and D) represent means ± SD (n = 3). *, P < 0.05; **, P < 0.01 (versus vector).
Figure 3.
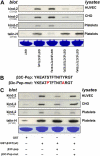
Distinct binding sites for kindlin-2 and talin in β3 CT. (A) Lysates of CHO cells, HUVECs, or out-dated platelets were incubated with GST or GST-fused β3 CT bearing the indicated mutations in the presence of glutathione-Sepharose. After washing, the precipitates were analyzed by SDS-PAGE. The loading of the GST proteins was assessed by Coomassie blue staining. The associated kindlin-2 or talin-H was detected in Western blots with anti-kindlin-2 or anti-talin-H. (B) Amino acid sequences of β3 CT C-terminal peptide corresponding to Y747-T762 and a mutant peptide with two loss-of-function mutations, S752P and Y759A. (C) The pull-down assay was performed in the presence of indicated peptides. The influence of these peptides on kindlin-2 or talin-H binding to β3 CT was evaluated by SDS-PAGE and Western blotting.
Figure 4.
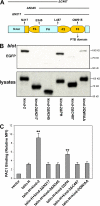
Both the N and C terminus of kindlin-2 are required for β3 CT association and support of talin-induced integrin activation. (A) Organization of predicated domains of kindlin-2 protein. The FERM domain is shown in yellow, in which the F2 subdomain is split by the PH domain. Deletion mutations from N terminus (ΔN) or C terminus (ΔC) are indicated. (B) The lysates of CHO cells transfected with EGFP-kindlin-2 with indicated mutations were used for pull-down assays. After incubating with GST fusion β3 CT (wild-type) and glutathione-Sepharose, kindlin-2 protein bound to the β3 CT was evaluated by SDS-PAGE and Western blotting using anti-GFP antibody. Kindlin-2 expression levels in lysates are also shown. (C) CHO cells expressing αIIbβ3 were transiently transfected with empty EGFP vector or cDNA encoding the indicated proteins. Binding of PAC1 to the different transfectants was assessed by FACS and relative MFI of PAC1 binding were calculated as described in Materials and methods. Error bars represent means ± SD (n = 3). **, P < 0.01 (versus talin-H).
Figure 5.
Endogenous kindlin-2 supports β3 integrin function in cells. (A) Subcellular localizations of kindlin-2 and β3 integrin. HUVECs spread on fibrinogen for 30 or 60 min were stained with the anti-kindlin-2 mAb and anti-β3 subunit polyclonal antibody followed by AlexaFluor 568 anti–mouse IgG and AlexaFluor 488 anti–rabbit IgG. Bar, 10 μm. (B) RNAi suppression of kindlin-2 expression in CHO cells. Expression of kindlin-2 in parental CHO cells (non-T), kindlin-2 siRNA (SiKind-2), or control RNA (SiControl) transfectants was analyzed by Western blotting with kindlin-2 or actin antibodies. (C) CHO cells expressing αIIbβ3 were transiently transfected with vector or talin-H, together with control RNAs (SiControl) or siRNAs targeting kindlin-2 (SiKind-2). The binding of PAC1 to the different transfectants was assessed by FACS and MFI of PAC1 binding was calculated. The error bars are means ± SD (n = 3). (D) RNAi suppression of kindlin-2 expression in HUVECs. (E and F) Non-transfected (Non-T) or HUVECs transfected with control RNAs (SiControl) or targeted siRNAs for Kindlin-2 (SiKind-2) were used in adhesion assays (E) or migration assays (F). The adherent or migrated cells were fixed, stained, and counted (10× objective). The error bars are means ± SD of three independent experiments. (G) HUVECs transfected with control RNAs (SiControl) or targeted siRNAs for kindlin-2 (SiKind-2) were stimulated with PMA, and adhesion to fibrinogen was measured. (H) Kindlin-2 as an integrin coactivator. Integrin activation depends on interaction of talin-H with the NPLY747 motif and the membrane-proximal clasping region. Kindlin-2 facilitates activation by associating with the C-terminal regions of β3 CT, involving the TS752T and NITY759 motifs.
Similar articles
- A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation.
Bouaouina M, Goult BT, Huet-Calderwood C, Bate N, Brahme NN, Barsukov IL, Critchley DR, Calderwood DA. Bouaouina M, et al. J Biol Chem. 2012 Mar 2;287(10):6979-90. doi: 10.1074/jbc.M111.330845. Epub 2012 Jan 10. J Biol Chem. 2012. PMID: 22235127 Free PMC article. - Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects.
Harburger DS, Bouaouina M, Calderwood DA. Harburger DS, et al. J Biol Chem. 2009 Apr 24;284(17):11485-97. doi: 10.1074/jbc.M809233200. Epub 2009 Feb 23. J Biol Chem. 2009. PMID: 19240021 Free PMC article. - Kindlin supports platelet integrin αIIbβ3 activation by interacting with paxillin.
Gao J, Huang M, Lai J, Mao K, Sun P, Cao Z, Hu Y, Zhang Y, Schulte ML, Jin C, Wang J, White GC, Xu Z, Ma YQ. Gao J, et al. J Cell Sci. 2017 Nov 1;130(21):3764-3775. doi: 10.1242/jcs.205641. Epub 2017 Sep 27. J Cell Sci. 2017. PMID: 28954813 Free PMC article. - Talin and Kindlin as Integrin-Activating Proteins: Focus on the Heart.
Chen C, Manso AM, Ross RS. Chen C, et al. Pediatr Cardiol. 2019 Oct;40(7):1401-1409. doi: 10.1007/s00246-019-02167-3. Epub 2019 Jul 31. Pediatr Cardiol. 2019. PMID: 31367953 Free PMC article. Review. - Kindlin: helper, co-activator, or booster of talin in integrin activation?
Ye F, Petrich BG. Ye F, et al. Curr Opin Hematol. 2011 Sep;18(5):356-60. doi: 10.1097/MOH.0b013e3283497f09. Curr Opin Hematol. 2011. PMID: 21730832 Review.
Cited by
- Integrin αIIbβ3 inside-out activation: an in situ conformational analysis reveals a new mechanism.
Kurtz L, Kao L, Newman D, Kurtz I, Zhu Q. Kurtz L, et al. J Biol Chem. 2012 Jun 29;287(27):23255-65. doi: 10.1074/jbc.M112.360966. Epub 2012 May 21. J Biol Chem. 2012. PMID: 22613710 Free PMC article. - Interaction of kindlin-2 with integrin β3 promotes outside-in signaling responses by the αVβ3 vitronectin receptor.
Liao Z, Kato H, Pandey M, Cantor JM, Ablooglu AJ, Ginsberg MH, Shattil SJ. Liao Z, et al. Blood. 2015 Mar 19;125(12):1995-2004. doi: 10.1182/blood-2014-09-603035. Epub 2015 Jan 13. Blood. 2015. PMID: 25587038 Free PMC article. - _β_2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function.
Bouti P, Webbers SDS, Fagerholm SC, Alon R, Moser M, Matlung HL, Kuijpers TW. Bouti P, et al. Front Immunol. 2021 Feb 18;11:619925. doi: 10.3389/fimmu.2020.619925. eCollection 2020. Front Immunol. 2021. PMID: 33679708 Free PMC article. Review. - Kindlin-2 mediates mechanotransduction in bone by regulating expression of Sclerostin in osteocytes.
Qin L, Fu X, Ma J, Lin M, Zhang P, Wang Y, Yan Q, Tao C, Liu W, Tang B, Chen D, Bai X, Cao H, Xiao G. Qin L, et al. Commun Biol. 2021 Mar 25;4(1):402. doi: 10.1038/s42003-021-01950-4. Commun Biol. 2021. PMID: 33767359 Free PMC article. - Whole-exome analysis of adolescents with low VWF and heavy menstrual bleeding identifies novel genetic associations.
Sadler B, Minard CG, Haller G, Gurnett CA, O'Brien SH, Wheeler A, Jain S, Sharma M, Zia A, Kulkarni R, Mullins E, Ragni MV, Sidonio R, Dietrich JE, Kouides PA, Di Paola J, Srivaths L. Sadler B, et al. Blood Adv. 2022 Jan 25;6(2):420-428. doi: 10.1182/bloodadvances.2021005118. Blood Adv. 2022. PMID: 34807970 Free PMC article.
References
- Byzova, T.V., C.K. Goldman, N. Pampori, K.A. Thomas, A. Bett, S.J. Shattil, and E.F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell. 6:851–860. - PubMed
- Chen, Y.-P., I. Djaffar, D. Pidard, B. Steiner, A.-M. Cieutat, J.P. Caen, and J.-P. Rosa. 1992. Ser-752→Pro mutation in the cytoplasmic domain of integrin β3 subunit and defective activation of platelet integrin αIIbβ3 (GPIIb-IIIa) in a variant of Glanzmann's thrombasthenia. Proc. Natl. Acad. Sci. USA. 89:10169–10173. - PMC - PubMed
- Chen, Y.P., T.E. O'Toole, J. Ylanne, J.P. Rosa, and M.H. Ginsberg. 1994. A point mutation in the integrin beta 3 cytoplasmic domain (S752→P) impairs bidirectional signaling through alpha IIb beta 3 (platelet glycoprotein IIb-IIIa). Blood. 84:1857–1865. - PubMed
- Dowling, J.J., E. Gibbs, M. Russell, D. Goldman, J. Minarcik, J.A. Golden, and E.L. Feldman. 2008. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ. Res. 102:423–431. - PubMed
- Eigenthaler, M., L. Hofferer, S.J. Shattil, and M.H. Ginsberg. 1997. A conserved sequence motif in the integrin beta3 cytoplasmic domain is required for its specific interaction with beta3-endonexin. J. Biol. Chem. 272:7693–7698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01HL073311/HL/NHLBI NIH HHS/United States
- R01 GM065188/GM/NIGMS NIH HHS/United States
- P01 HL073311/HL/NHLBI NIH HHS/United States
- R01 GM062823/GM/NIGMS NIH HHS/United States
- GM65188/GM/NIGMS NIH HHS/United States
- GM62823/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials