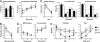A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition - PubMed (original) (raw)
A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition
Mirna Kvajo et al. Proc Natl Acad Sci U S A. 2008.
Abstract
DISC1 is a strong candidate susceptibility gene for schizophrenia, bipolar disorder, and depression. Using a mouse strain carrying an endogenous Disc1 orthologue engineered to model the putative effects of the disease-associated chromosomal translocation we demonstrate that impaired Disc1 function results in region-specific morphological alterations, including alterations in the organization of newly born and mature neurons of the dentate gyrus. Field recordings at CA3/CA1 synapses revealed a deficit in short-term plasticity. Using a battery of cognitive tests we found a selective impairment in working memory (WM), which may relate to deficits in WM and executive function observed in individuals with schizophrenia. Our results implicate malfunction of neural circuits within the hippocampus and medial prefrontal cortex and selective deficits in WM as contributing to the genetic risk conferred by this gene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
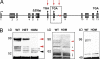
Structure of the targeted Disc1 allele and its consequences on protein production and brain morphology. (A) A multiply compromised Disc1 allele. The modified Disc1 allele carries (i) the 129 25-bp deletion variant (2) in exon 6 that results in the introduction of a premature termination codon in exon 7; (ii) a termination codon in exon 8 introduced by homologous recombination; and (iii) a polyadenylation signal in intron 8 introduced by homologous recombination, which results in the generation of a polyadenylated truncated transcript (2). The modifications in the Disc1 allele are indicated by red arrows. (B) Western blot analysis of brain homogenates from WT and mutant Disc1 mice. (Left) The ≈98-kDa band, corresponding to the predicted full-length proteins (L and Lv forms,
) is undetectable in early postnatal day 2 brain lysates from HOM mutant Disc1 mice (top red arrowhead) analyzed with the N-terminal antibody. The same band is reduced by about half in HET mutant mice. A band consistent with the predicted C-terminally truncated protein product (58 kDa) (black arrowhead) is detectable in lysates from HET and HOM mutant Disc1 mice, but not WT littermates. This band is weak, indicating that the truncated protein missing the C-terminal portion is relatively unstable. Detection of this product proves unequivocally the specificity of our antibody. A ≈70-kDa band (bottom red arrowhead) is undetectable in HOM mutant Disc1 mice and reduced by half in HET mutant Disc1 mice. Two other weak low molecular weight bands appear unaffected by the mutation and may represent shorter N-terminal isoforms (2) (terminating before exon 6) or nonspecific signals (asterisks). Such short (putative) isoforms are expected to be produced and persist in the brains of the affected members of the Scottish family. (Center) Analysis with a C-terminal antibody. Red arrowheads indicate the major Disc1 isoforms at ≈98 and 70 kDa, which are missing in HOM mice. As predicted, no truncated protein is detected with this antibody. Asterisks indicate likely nonspecific cross-reactivity. (Right) Western blot of adult HPC lysates probed with the N-terminal antibody.
Fig. 2.
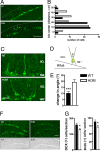
Altered positioning and numbers of immature neurons. (A and B) Distribution of immature neurons in the GCL. (A) Representative images of DCX-positive neurons from HOM mutant Disc1 mice and their WT littermates. Note the presence of DCX-positive neurons in the deeper layer of the GCL (arrows) of mutant mice. The dotted line represents the outer edge of the GCL. (B) Quantification of neuronal distribution performed by using the χ2 test in a contingency analysis. n = 54 cells were analyzed for each genotype. (C–E) Dendritic orientation of immature neurons. (C) Representative images of DCX-positive neurons from HOM mutant Disc1 mice and their WT littermates. (D and E) Quantification was performed by defining the angle of orientation (θ) for each apical dendrite (D) and calculating the mean change in θ (E). (F and G) Quantification of proliferating cells in the CGL. (F) Representative images of DCX-positive immature neurons (Upper) and BrdU-labeled neural precursor cells (Lower) in the DG of HOM mutant Disc1 mice as compared with their WT littermates. (G) Quantification of DCX-positive immature neurons (Left) and BrdU-labeled neural precursor cells (Right). Values represent mean ± SEM. ∗, P < 0.05; ∗∗∗, P < 0.0001. (Scale bars: A and F, 100 μm; C, 25 μm.)
Fig. 3.
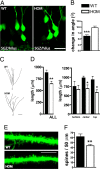
Cytoarchitectural alterations in mature granule cells. (A and B) Dendritic orientation of DG granule cells. (A) Representative images of GFP-labeled mature granule cells. (B) Quantification of dendritic misorientation. (C and D) Dendritic length of DG granule cells. (C) Representative tracings of GFP-labeled mature granule cells. (D) Quantification of total dendritic length plotted against their position in the GCL. WT/HOM (n): all (14/17); top (4/6); center (6/7); bottom (4/4). (E and F) Dendritic spine density of DG granule cells. Representative images of dendritic spines (E) and quantification of their numbers (F). Values represent mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.001; ∗∗∗, P < 0.0001. (Scale bars: A, 25 μm; C, 50 μm; E, 5 μm.)
Fig. 4.
Synaptic transmission and plasticity. (A) LTP in WT (n = 17, 7) and HOM (n = 10, 6) mice. There was a significant difference in the degree of potentiation of fEPSPs over time (min) (two-way repeated measures ANOVA; P < 0.05). Posthoc testing showed that 14 min immediately after tetanization the magnitude of potentiation in the HOM mutant Disc1 mice was significantly lower than in WT controls. By contrast, the degree of LTP observed was unaffected by the mutation 1 h after tetanization. (B) Example traces of field EPSPs show responses before (solid line) and immediately after (interrupted line) tetanization.
Fig. 5.
A mutation in Disc1 leads to specific pattern of cognitive deficits. (A) Fear conditioning. (Left) Conditioned freezing during the cued test (n = 9 WT, 9 HOM). Percent of time freezing during the 60 s before tone presentation (pretone) and during the 20-s tone presentation (tone). (Right) Percent time freezing during the 4 min of the contextual test. (B) Novel object recognition. Although the ability to recognize a familiar object significantly decreased with time, there were no differences between genotypes (n = 9 WT, 9 HOM). (C) Morris water maze. (Left) Latency to reach a visible platform did not differ between genotypes (n = 9 WT, 9 HOM). (Center and Right) Both genotypes spent more time in the target quadrant during the probe trials. (D) Win-shift task. Both genotypes made comparable numbers of within-phase (Left) and across-phase (Right) errors (n = 9 WT, 9 HOM). (E) Two-choice DNMP task. Both HET and HOM mutant Disc1 mice made significantly more errors during training (Left) and testing (Right; n = 10 WT, 11 HET, 11 HOM). Values represent mean ± SEM. n.s., not significant; ∗, P < 0.05.
Similar articles
- Alteration of Neuronal Excitability and Short-Term Synaptic Plasticity in the Prefrontal Cortex of a Mouse Model of Mental Illness.
Crabtree GW, Sun Z, Kvajo M, Broek JA, Fénelon K, McKellar H, Xiao L, Xu B, Bahn S, O'Donnell JM, Gogos JA. Crabtree GW, et al. J Neurosci. 2017 Apr 12;37(15):4158-4180. doi: 10.1523/JNEUROSCI.4345-15.2017. Epub 2017 Mar 10. J Neurosci. 2017. PMID: 28283561 Free PMC article. - Disrupted-in-schizophrenia1 (DISC1) L100P mutation alters synaptic transmission and plasticity in the hippocampus and causes recognition memory deficits.
Cui L, Sun W, Yu M, Li N, Guo L, Gu H, Zhou Y. Cui L, et al. Mol Brain. 2016 Oct 12;9(1):89. doi: 10.1186/s13041-016-0270-y. Mol Brain. 2016. PMID: 27729083 Free PMC article. - Phenotypic characterization of C57BL/6J mice carrying the Disc1 gene from the 129S6/SvEv strain.
Juan LW, Liao CC, Lai WS, Chang CY, Pei JC, Wong WR, Liu CM, Hwu HG, Lee LJ. Juan LW, et al. Brain Struct Funct. 2014 Jul;219(4):1417-31. doi: 10.1007/s00429-013-0577-8. Epub 2013 May 21. Brain Struct Funct. 2014. PMID: 23689501 - Schizophrenia in translation: disrupted in schizophrenia (DISC1): integrating clinical and basic findings.
Roberts RC. Roberts RC. Schizophr Bull. 2007 Jan;33(1):11-5. doi: 10.1093/schbul/sbl063. Epub 2006 Nov 30. Schizophr Bull. 2007. PMID: 17138582 Free PMC article. Review. - DISC1 in schizophrenia: genetic mouse models and human genomic imaging.
Johnstone M, Thomson PA, Hall J, McIntosh AM, Lawrie SM, Porteous DJ. Johnstone M, et al. Schizophr Bull. 2011 Jan;37(1):14-20. doi: 10.1093/schbul/sbq135. Epub 2010 Dec 13. Schizophr Bull. 2011. PMID: 21149852 Free PMC article. Review.
Cited by
- Progression of behavioral deficits during periadolescent development differs in female and male DISC1 knockout rats.
Glenn MJ, Batallán Burrowes AA, Yu W, Blackmer-Raynolds L, Norchi A, Doak AL. Glenn MJ, et al. Genes Brain Behav. 2022 Jan;21(1):e12741. doi: 10.1111/gbb.12741. Epub 2021 Jun 21. Genes Brain Behav. 2022. PMID: 33960643 Free PMC article. - DISC1 genetics, biology and psychiatric illness.
Thomson PA, Malavasi EL, Grünewald E, Soares DC, Borkowska M, Millar JK. Thomson PA, et al. Front Biol (Beijing). 2013 Feb 1;8(1):1-31. doi: 10.1007/s11515-012-1254-7. Front Biol (Beijing). 2013. PMID: 23550053 Free PMC article. - From "directed differentiation" to "neuronal induction": modeling neuropsychiatric disease.
Ho SM, Topol A, Brennand KJ. Ho SM, et al. Biomark Insights. 2015 Apr 27;10(Suppl 1):31-41. doi: 10.4137/BMI.S20066. eCollection 2015. Biomark Insights. 2015. PMID: 26045654 Free PMC article. Review. - Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1.
Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Jaaro-Peled H, et al. Trends Neurosci. 2009 Sep;32(9):485-95. doi: 10.1016/j.tins.2009.05.007. Epub 2009 Aug 26. Trends Neurosci. 2009. PMID: 19712980 Free PMC article. Review. - The telomeric part of the human chromosome 21 from Cstb to Prmt2 is not necessary for the locomotor and short-term memory deficits observed in the Tc1 mouse model of Down syndrome.
Duchon A, Pothion S, Brault V, Sharp AJ, Tybulewicz VL, Fisher EM, Herault Y. Duchon A, et al. Behav Brain Res. 2011 Mar 1;217(2):271-81. doi: 10.1016/j.bbr.2010.10.023. Epub 2010 Oct 31. Behav Brain Res. 2011. PMID: 21047530 Free PMC article.
References
- Millar JK, et al. Disruption of two novel genes by a translocation cosegregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. - PubMed
- Arguello PA, Gogos JA. Modeling madness in mice: One piece at a time. Neuron. 2006;52:179–196. - PubMed
- Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: Emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–233. - PubMed
- Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH67068/MH/NIMH NIH HHS/United States
- MH77235/MH/NIMH NIH HHS/United States
- P01 NS048120/NS/NINDS NIH HHS/United States
- R01 MH077235/MH/NIMH NIH HHS/United States
- R01 MH080234/MH/NIMH NIH HHS/United States
- R01 MH067068/MH/NIMH NIH HHS/United States
- MH080234/MH/NIMH NIH HHS/United States
- T32 GM008224/GM/NIGMS NIH HHS/United States
- T32GM008224/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous