An endogenous TNF-alpha antagonist induced by splice-switching oligonucleotides reduces inflammation in hepatitis and arthritis mouse models - PubMed (original) (raw)
An endogenous TNF-alpha antagonist induced by splice-switching oligonucleotides reduces inflammation in hepatitis and arthritis mouse models
Maria A Graziewicz et al. Mol Ther. 2008 Jul.
Abstract
Tumor necrosis factor-alpha (TNF-alpha) is a key mediator of inflammatory diseases, including rheumatoid arthritis (RA), and anti-TNF-alpha drugs such as etanercept are effective treatments. Splice-switching oligonucleotides (SSOs) are a new class of drugs designed to induce therapeutically favorable splice variants of targeted genes. In this work, we used locked nucleic acid (LNA)-based SSOs to modulate splicing of TNF receptor 2 (TNFR2) pre-mRNA. The SSO induced skipping of TNFR2 exon 7, which codes the transmembrane domain (TM), switching endogenous expression from the membrane-bound, functional form to a soluble, secreted form (Delta7TNFR2). This decoy receptor protein accumulated in the circulation of treated mice, antagonized TNF-alpha, and altered disease in two mouse models: TNF-alpha-induced hepatitis and collagen-induced arthritis (CIA). This is the first report of upregulation of the endogenous, circulating TNF-alpha antagonist by oligonucleotide-induced splicing modulation.
Figures
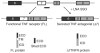
Figure 1. The Δ7TNFR2 splice variant
The full-length (FL), functional, membrane-bound tumor necrosis factor receptor 2 (TNFR2) is translated from messenger RNA (mRNA) containing all 10 coding exons. Targeting of exon 7 in pre-mRNA with LNA SSOs (black bars) induces a soluble isoform, Δ7TNFR2, that lacks a transmembrane domain (TM), retains the extracellular and intracellular domains (ECD and ICD, respectively) and is secreted by cells. Importantly, the shed form of soluble TNFR2 contains only the ECD. Black, extracellular domain; white, transmembrane domain; gray, intracellular domain. LNA, locked nucleic acid; SSOs, splice-switching oligonucleotides.
Figure 2. Induction of Δ7TNFR2 by locked nucleic acid (LNA) SSO3274
(a) LNA splice-switching oligonucleotides (SSOs) targeted to mouse tumor necrosis factor receptor 2 (TNFR2) pre-mRNA from 20 bp upstream to 20 bp downstream and including exon 7. Thick line, exon; thin line, intron; short lines, LNA SSOs. L929 cells were transfected with the indicated SSOs at a final concentration of 50 nmol/l. After 24 hours, cells were lysed, RNA was isolated and analyzed for splice switching by reverse transcription–PCR (RT-PCR). FL, full-length TNFR2 messenger RNA (mRNA); Δ7, Δ7TNFR2 mRNA splice variant. (b) Mice (n = 5 per group) were injected intraperitoneally with SSO3274 or control SSO3272 at 25 mg/kg/day once daily for 5 days. Serum was collected 4 days before injections began (0) and at the indicated number of days after the last SSO injection (gray). Top, samples were analyzed by enzyme-linked immunosorbent assay. Bottom, mice were killed at indicated days and total liver RNA was analyzed by RT-PCR for TNFR2 pre-mRNA splice switching. Each lane represents analysis of liver RNA from a single treated mouse. Data for days 10 and 27 are from separate experiments.
Figure 3. Anti–TNF-α activity of Δ7TNFR2 protein
(a) LNA SSOs were administered to mice as described at 25 mg/kg/day once daily for 10 days. The serum was collected 5 or 27 days after the last injection, diluted to 10% and applied to L929 in the presence of actinomycin D and TNF-α (ActD/TNF-α). After 24 hours, cell viability was determined using MTS assay and compared with controls. Ctr, cells treated with 10% serum from untreated mice and no ActD/TNF; TNF, cells treated with 10% serum from untreated mice and ActD/TNF; TNF +, cells treated with 10% serum from mice treated with the indicated SSO and ActD/TNF. TNF + 3274 (n = 3), all other (n = 1). Note that serum from SSO3272, 3083, and untreated mice yielded a reproducible lack of protection from TNF-α. (b) Anti-TNFR2 antibodies (Abs) neutralize the anti–TNF-α effect of Δ7TNFR2. The L929 cytotoxicity assay was performed as described in a, except that the indicated concentrations of an anti-TNFR2 Ab was added at the indicated concentrations. Ctr, L929 cell receiving no ActD/TNF-α, anti-TNFR2 Ab, or Δ7TNFR2; Ab, cells receiving 200 ng/ml anti-TNFR2 Ab only; TNF, cells receiving ActD/TNF-α only. (c) Half maximal effective concentration (EC50) values calculated from a (see Materials and Methods). LNA, locked nucleic acid; SSO, splice-switching oligonucleotide; TNF-α, tumor necrosis factor-α; TNFR2, TNF receptor 2.
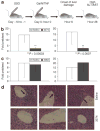
Figure 4. Tumor necrosis factor-α (TNF-α)-induced hepatitis model
(a) Mice were treated intraperitoneally with saline, control SSO3083 (see Figure 2a), or Δ7TNFR2 inducing SSO3274 at 25 mg/kg once daily for 10 days. After 24 hours (day 0, hour 0), mice were injected with 20 mg galactosamine (GalN) followed by 3 ng TNF-α 20 minutes later, which induces an acute inflammatory response in the liver. (b) The extent of hepatocyte damage caused by the inflammatory insult is reflected by increased levels of liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), released by hour 28 into the blood stream of control, saline-injected, mice (white bars). Treatment with locked nucleic acid SSO3274 (black bars) prevents liver damage and enzyme release. (c) Control SSO3083 (black bars) showed no preventative effect. Saline treatment (n = 15); splice-switching oligonucleotide (SSO) treatment (n = 10). P values for saline versus control SSO3083 in ALT or AST were P > 0.2. (d) Livers from mice treated as in b were removed at the time of killing (28 hours), imbedded in paraffin and stained with hematoxylin & eosin. Arrows, sites of inflammatory infiltrate. TNFR2, TNF receptor 2.
Figure 5. Locked nucleic acid (LNA) SSO3274 activity in collagen-induced arthritis (CIA) mice
(a) Time course of CIA model. Mice (n = 10) were treated with either saline from day 11–20 (saline) or 25 mg/kg/day of SSO3274 from day 11–15 or 11–20 (SSO 5D and 10D, respectively). (b) Serum was collected from mice on the indicated day of the experiment and analyzed by Δ7TNFR2 specific enzyme-linked immunosorbent assay, or (c) anti–TNF-α L929 cytotoxicity assay. (d) Administration of LNA SSO3274 for 10 days delayed and reduced the onset and extent of the course of the disease as measured by paw swelling or (e) clinical index. Measurements in d and e were performed as described in Materials and Methods. SSO, splice-switching oligonucleotide; TNF-α, tumor necrosis factor-α; TNFR2, TNF receptor 2.
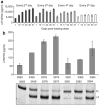
Figure 6. Therapeutic potential of Δ7TNFR2 inducing splice-switching oligonucleotide (SSO)
(a) Mice were injected with a loading dose of 5 days of once daily 25 mg/kg/day SSO3274, followed by a single 25 mg/kg maintenance dose every 2nd, 3rd, 4th, or 5th day for 37 days. Δ7TNFR2 was measured in serum samples as in previous figures. (b) The indicated SSO was delivered to primary human hepatocytes by cationic lipid transfection at a final concentration of ∼30 nmol/l. Total RNA and extracellular media were collected 72 hours after transfection, and total RNA was analyzed by reverse transcription–PCR and medium was analyzed by enzyme-linked immunosorbent assay (ELISA) for Δ7TNFR2 messenger RNA and protein, respectively. Note that this ELISA detects both the shed form and the Δ7TNFR2 form, thus control (3083)-treated cells show a small background of shed tumor necrosis factor receptor 2 (TNFR2) extracellular domain. FL, full length.
Similar articles
- Design and In Vitro Evaluation of Splice-Switching Oligonucleotides Bearing Locked Nucleic Acids, Amido-Bridged Nucleic Acids, and Guanidine-Bridged Nucleic Acids.
Shimo T, Nakatsuji Y, Tachibana K, Obika S. Shimo T, et al. Int J Mol Sci. 2021 Mar 29;22(7):3526. doi: 10.3390/ijms22073526. Int J Mol Sci. 2021. PMID: 33805378 Free PMC article. - An Endogenous TNF-α Antagonist Induced by Splice-switching Oligonucleotides Reduces Inflammation in Hepatitis and Arthritis Mouse Models.
Graziewicz MA, Tarrant TK, Buckley B, Roberts J, Fulton L, Hansen H, Ørum H, Kole R, Sazani P. Graziewicz MA, et al. Mol Ther. 2008 Jul;16(7):1316-1322. doi: 10.1038/mt.2008.85. Epub 2016 Dec 8. Mol Ther. 2008. PMID: 28178484 - Design and evaluation of locked nucleic acid-based splice-switching oligonucleotides in vitro.
Shimo T, Tachibana K, Saito K, Yoshida T, Tomita E, Waki R, Yamamoto T, Doi T, Inoue T, Kawakami J, Obika S. Shimo T, et al. Nucleic Acids Res. 2014 Jul;42(12):8174-87. doi: 10.1093/nar/gku512. Epub 2014 Jun 16. Nucleic Acids Res. 2014. PMID: 24935206 Free PMC article. - Alteration of the gut microbiota in tumor necrosis factor-α antagonist-treated collagen-induced arthritis mice.
Wang B, He Y, Tang J, Ou Q, Lin J. Wang B, et al. Int J Rheum Dis. 2020 Apr;23(4):472-479. doi: 10.1111/1756-185X.13802. Epub 2020 Feb 26. Int J Rheum Dis. 2020. PMID: 32100456 - Therapeutic potential of splice-switching oligonucleotides.
Bauman J, Jearawiriyapaisarn N, Kole R. Bauman J, et al. Oligonucleotides. 2009 Mar;19(1):1-13. doi: 10.1089/oli.2008.0161. Oligonucleotides. 2009. PMID: 19125639 Free PMC article. Review.
Cited by
- Targeting RNA splicing for disease therapy.
Havens MA, Duelli DM, Hastings ML. Havens MA, et al. Wiley Interdiscip Rev RNA. 2013 May-Jun;4(3):247-66. doi: 10.1002/wrna.1158. Epub 2013 Mar 19. Wiley Interdiscip Rev RNA. 2013. PMID: 23512601 Free PMC article. Review. - mRNA transcript diversity creates new opportunities for pharmacological intervention.
Barrie ES, Smith RM, Sanford JC, Sadee W. Barrie ES, et al. Mol Pharmacol. 2012 May;81(5):620-30. doi: 10.1124/mol.111.076604. Epub 2012 Feb 7. Mol Pharmacol. 2012. PMID: 22319206 Free PMC article. Review. - Targeting eosinophils in allergy, inflammation and beyond.
Fulkerson PC, Rothenberg ME. Fulkerson PC, et al. Nat Rev Drug Discov. 2013 Feb;12(2):117-29. doi: 10.1038/nrd3838. Epub 2013 Jan 21. Nat Rev Drug Discov. 2013. PMID: 23334207 Free PMC article. Review. - Design and In Vitro Evaluation of Splice-Switching Oligonucleotides Bearing Locked Nucleic Acids, Amido-Bridged Nucleic Acids, and Guanidine-Bridged Nucleic Acids.
Shimo T, Nakatsuji Y, Tachibana K, Obika S. Shimo T, et al. Int J Mol Sci. 2021 Mar 29;22(7):3526. doi: 10.3390/ijms22073526. Int J Mol Sci. 2021. PMID: 33805378 Free PMC article. - Directing HER4 mRNA expression towards the CYT2 isoform by antisense oligonucleotide decreases growth of breast cancer cells in vitro and in vivo.
Nielsen TO, Sorensen S, Dagnæs-Hansen F, Kjems J, Sorensen BS. Nielsen TO, et al. Br J Cancer. 2013 Jun 11;108(11):2291-8. doi: 10.1038/bjc.2013.247. Epub 2013 May 21. Br J Cancer. 2013. PMID: 23695025 Free PMC article.
References
- Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003;2:736–746. - PubMed
- Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2006;63:2451–2465. - PubMed
- Lainez B, Fernandez-Real JM, Romero X, Esplugues E, Canete JD, Ricart W, et al. Identification and characterization of a novel spliced variant that encodes human soluble tumor necrosis factor receptor 2. Int Immunol. 2004;16:169–177. - PubMed
- Sazani P, Graziewicz M, Kole R. Splice switching oligonucleotides as potential therapeutics. In: Crooke ST, editor. Antisense Drug Technology: Principles, Strategies, and Applications. 2nd. CRC Press; Boca Raton, FL: 2007. pp. 89–114.
Publication types
MeSH terms
Substances
Grants and funding
- P01-GM059299/GM/NIGMS NIH HHS/United States
- P01 GM059299-090001/GM/NIGMS NIH HHS/United States
- 41AR054686-01/AR/NIAMS NIH HHS/United States
- P01 GM059299/GM/NIGMS NIH HHS/United States
- R41 AR054686/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical