The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line - PubMed (original) (raw)
The endosymbiont Wolbachia pipientis induces the expression of host antioxidant proteins in an Aedes albopictus cell line
Lesley J Brennan et al. PLoS One. 2008.
Abstract
Wolbachia are obligate intracellular bacteria which commonly infect arthropods. They are maternally inherited and capable of altering host development, sex determination, and reproduction. Reproductive manipulations include feminization, male-killing, parthenogenesis, and cytoplasmic incompatibility. The mechanism by which Wolbachia avoid destruction by the host immune response is unknown. Generation of antimicrobial peptides (AMPs) and reactive oxygen species (ROS) by the host are among the first lines of traditional antimicrobial defense. Previous work shows no link between a Wolbachia infection and the induction of AMPs. Here we compare the expression of protein in a cell line naturally infected with Wolbachia and an identical cell line cured of the infection through the use of antibiotics. Protein extracts of each cell line were analyzed by two dimensional gel electrophoresis and LC/MS/MS. Our results show the upregulation of host antioxidant proteins, which are active against ROS generated by aerobic cell metabolism and during an immune response. Furthermore, flow cytometric and microscopic analysis demonstrates that ROS production is significantly greater in Wolbachia-infected mosquito cells and is associated with endosymbiont-containing vacuoles located in the host cell cytoplasm. This is the first empirical data supporting an association between Wolbachia and the insect antioxidant system.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Wolbachia stably infects Aa23 cells and can be cured by antibiotic treatment.
(A) PCR analysis using Wolbachia wsp primers (top) and arthropod 28S primers (bottom) of Aa23 cells treated with 10 ug/ml rifampicin for seven passages. Lane L: molecular ladder. Lane 1: stably infected Aa23 cells. Lanes 2 through 8: cells treated with rifampicin for 1, 2, 3, 4, 5, 6, and 7 passages. Lane 9: negative control. (B) Aa23 cells stably infected with Wolbachia (I) and Aa23 cells cured of Wolbachia using rifampicin (II). Bar, 100 µm.
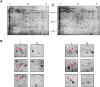
Figure 2. 2-D Page of _Wolbachia_-infected and uninfected Aa23 cells.
(A) Identification of proteins unique to _Wolbachia-_infected Aa23 cells. Approximately 750 ug of protein extract from an Aa23 cell line stably infected with Wolbachia (I) and a parallel cell line cured of a Wolbachia infection (II) were analyzed. Proteins expressed only in the presence of a Wolbachia infection (ID #1–6) are identified. (B) Gel sections showing proteins selected for LC/MS/MS analysis.
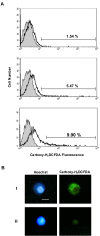
Figure 3. Analysis of ROS formation in Wolbachia -infected and uninfected Aa23 cells.
(A) Flow cytometric analysis of Wolbachia –infected and uninfected Aa23 cells using the fluorescent ROS marker carboxy-H2DCFDA. Histograms representative of three replicates are shown. The negative control (shaded) consists of unlabeled cells. Test samples (black lines) include: uninfected Aa23 cells (top panel), uninfected Aa23 cells induced to produce ROS using TBHP (middle panel), and infected Aa23 cells (bottom panel). Carboxy-H2DCFDA positive cells are represented on each histogram. (B) Microscopic analysis of _Wolbachia_-infected (I) and uninfected (II) Aa23 cells. Hoechst stain was used to label DNA (left panel). Carboxy-H2DCFDA was used to label ROS (right panel). Bar, 10 µm.
Similar articles
- Wolbachia replication and host cell division in Aedes albopictus.
Ruang-areerate T, Kittayapong P, McGraw EA, Baimai V, O'Neill SL. Ruang-areerate T, et al. Curr Microbiol. 2004 Jul;49(1):10-2. doi: 10.1007/s00284-003-4245-8. Curr Microbiol. 2004. PMID: 15297923 - Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus.
Dobson SL, Rattanadechakul W, Marsland EJ. Dobson SL, et al. Heredity (Edinb). 2004 Aug;93(2):135-42. doi: 10.1038/sj.hdy.6800458. Heredity (Edinb). 2004. PMID: 15127087 - Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta).
Moretti R, Calvitti M. Moretti R, et al. Med Vet Entomol. 2013 Dec;27(4):377-86. doi: 10.1111/j.1365-2915.2012.01061.x. Epub 2012 Nov 22. Med Vet Entomol. 2013. PMID: 23171418 - The Biochemistry of Cytoplasmic Incompatibility Caused by Endosymbiotic Bacteria.
Chen H, Zhang M, Hochstrasser M. Chen H, et al. Genes (Basel). 2020 Jul 25;11(8):852. doi: 10.3390/genes11080852. Genes (Basel). 2020. PMID: 32722516 Free PMC article. Review. - Wolbachia: endosymbiont of onchocercid nematodes and their vectors.
Manoj RRS, Latrofa MS, Epis S, Otranto D. Manoj RRS, et al. Parasit Vectors. 2021 May 7;14(1):245. doi: 10.1186/s13071-021-04742-1. Parasit Vectors. 2021. PMID: 33962669 Free PMC article. Review.
Cited by
- Influence of Wolbachia on host gene expression in an obligatory symbiosis.
Kremer N, Charif D, Henri H, Gavory F, Wincker P, Mavingui P, Vavre F. Kremer N, et al. BMC Microbiol. 2012 Jan 18;12 Suppl 1(Suppl 1):S7. doi: 10.1186/1471-2180-12-S1-S7. BMC Microbiol. 2012. PMID: 22376153 Free PMC article. - Infection by chikungunya virus modulates the expression of several proteins in Aedes aegypti salivary glands.
Tchankouo-Nguetcheu S, Bourguet E, Lenormand P, Rousselle JC, Namane A, Choumet V. Tchankouo-Nguetcheu S, et al. Parasit Vectors. 2012 Nov 15;5:264. doi: 10.1186/1756-3305-5-264. Parasit Vectors. 2012. PMID: 23153178 Free PMC article. - Repurposing of approved drugs from the human pharmacopoeia to target Wolbachia endosymbionts of onchocerciasis and lymphatic filariasis.
Johnston KL, Ford L, Umareddy I, Townson S, Specht S, Pfarr K, Hoerauf A, Altmeyer R, Taylor MJ. Johnston KL, et al. Int J Parasitol Drugs Drug Resist. 2014 Sep 16;4(3):278-86. doi: 10.1016/j.ijpddr.2014.09.001. eCollection 2014 Dec. Int J Parasitol Drugs Drug Resist. 2014. PMID: 25516838 Free PMC article. - Impact of Wolbachia on oxidative stress sensitivity in the parasitic wasp Asobara japonica.
Monnin D, Kremer N, Desouhant E, Vavre F. Monnin D, et al. PLoS One. 2017 Apr 20;12(4):e0175974. doi: 10.1371/journal.pone.0175974. eCollection 2017. PLoS One. 2017. PMID: 28426794 Free PMC article. - The oxidizing agent, paraquat, is more toxic to Wolbachia than to mosquito host cells.
Fallon AM, Kurtz CM, Carroll EM. Fallon AM, et al. In Vitro Cell Dev Biol Anim. 2013 Aug;49(7):501-7. doi: 10.1007/s11626-013-9634-0. Epub 2013 May 30. In Vitro Cell Dev Biol Anim. 2013. PMID: 23719839 Free PMC article.
References
- Harris HL, Braig HR. Sperm chromatin remodelling and Wolbachia-induced cytoplasmic incompatibility in Drosophila. Biochem Cell Biol. 2003;81:229–240. - PubMed
- Hurst GDD, Jiggins FM, Graf von der Schulenburg JH, Bertrand D, West SA, et al. Male-killing Wolbachia in two species of insect. Proc R Soc Lond B Biol Sci. 1999;266:735–740.
- Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc R Soc Lond B Biol Sci. 1992;250:91–98. - PubMed
- Stouthamer R, Breeuwer JAJ, Luck RF, Werren JH. Molecular identification of microorganisms associated with parthenogenesis. Nature. 1993;361:66–68. - PubMed
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources