Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics - PubMed (original) (raw)
Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics
Leonardo G Trabuco et al. Structure. 2008 May.
Abstract
A novel method to flexibly fit atomic structures into electron microscopy (EM) maps using molecular dynamics simulations is presented. The simulations incorporate the EM data as an external potential added to the molecular dynamics force field, allowing all internal features present in the EM map to be used in the fitting process, while the model remains fully flexible and stereochemically correct. The molecular dynamics flexible fitting (MDFF) method is validated for available crystal structures of protein and RNA in different conformations; measures to assess and monitor the fitting process are introduced. The MDFF method is then used to obtain high-resolution structures of the E. coli ribosome in different functional states imaged by cryo-EM.
Figures
Figure 1
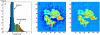
Reconstruction of the E. coli ribosome from cryo-EM data at ∼12.8 Å resolution (K.M., L.G.T., E.V., A. Zavialov, M. Ehrenberg, K.S., and J.F., in preparation). (A) A density histogram shows two distinct peaks pertaining to the solvent and macromolecule; (B) 2-D slice of the density; (C) 2-D slice of the density after clamping values below the average, thus homogenizing the density corresponding to the solvent surrounding the macromolecule and the bulk solvent.
Figure 2
Harmonic restraints applied to base-paired RNA residues. (A) RNA interaction edges for both purines (adenine is shown of the left) and pyrimidines (cytosine is shown on the right), according to Leontis and Westhof (1998); (B) dihedral angles, and the two interatomic distances (dashed lines) to which harmonic restraints are applied.
Figure 3
Validation of the MDFF method using X-ray structures in two conformations. (A) Acethyl-CoA synthase; (B) 16S rRNA (only the head is shown for clarity, since it is the only region where the two conformations differ significantly). The target structures and simulated maps are shown in gray, whereas the initial and final fitted structures are shown in green (top) and colored by backbone RMSD per residue with respect to the target structures (bottom; color scales in Å). The final structures correspond to fittings into 10-Å simulated maps generated from the target structures. Movies of the fittings are included in Supplemental Data S4.
Figure 4
Fitting into the TC-bound ribosome cryo-EM map at 6.7-Å resolution by means of MDFF. (A) Overview of the all-atom ribosome structure fitted into the 6.7-Å map, with a close view into the decoding center (inset). (B) Conformation of tRNA in the A/T site. The crystal structure from the free TC used as a starting point for the fitting (PDB 1OB2, unpublished data) is shown in red; the A/T tRNA model obtained by applying the MDFF method to the 6.7-Å map is shown in blue; the A/T tRNA model previously obtained using a 9.0-Å map constructed by interpolating two manual fittings of tRNA (PDB 1OB2) is shown in green (Valle et al., 2003). (C) Conformation of tRNA in the A/T site (blue) compared to a partial crystal structure of the A-site tRNA (Selmer et al., 2006) (red). The crystal structure from the free TC used as a starting point for the fitting (PDB 1OB2, unpublished data) is shown on the left; the A/T tRNA model obtained by applying the MDFF method to the 6.7-Å map is shown on the right. (D) Conformational dynamics of the GTPase-associated center. Shown are differences in the conformation of the GTPase-associated center between the TC-bound ribosome (EM map at 6.7-Å resolution, top), and the accommodated ribosome (EM map at 9-Å resolution, bottom). Rigid-body docked structures into the corresponding maps, used as initial coordinates for flexible fitting, are shown on the left; flexibly fitted structures are shown on the right.
Similar articles
- Molecular dynamics flexible fitting: a practical guide to combine cryo-electron microscopy and X-ray crystallography.
Trabuco LG, Villa E, Schreiner E, Harrison CB, Schulten K. Trabuco LG, et al. Methods. 2009 Oct;49(2):174-80. doi: 10.1016/j.ymeth.2009.04.005. Epub 2009 May 4. Methods. 2009. PMID: 19398010 Free PMC article. - Structure of the E. coli ribosome-EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM.
Fischer N, Neumann P, Konevega AL, Bock LV, Ficner R, Rodnina MV, Stark H. Fischer N, et al. Nature. 2015 Apr 23;520(7548):567-70. doi: 10.1038/nature14275. Epub 2015 Feb 23. Nature. 2015. PMID: 25707802 - The 3D arrangement of the 23 S and 5 S rRNA in the Escherichia coli 50 S ribosomal subunit based on a cryo-electron microscopic reconstruction at 7.5 A resolution.
Mueller F, Sommer I, Baranov P, Matadeen R, Stoldt M, Wöhnert J, Görlach M, van Heel M, Brimacombe R. Mueller F, et al. J Mol Biol. 2000 Apr 21;298(1):35-59. doi: 10.1006/jmbi.2000.3635. J Mol Biol. 2000. PMID: 10756104 - Cryo-electron microscopy as an investigative tool: the ribosome as an example.
Frank J. Frank J. Bioessays. 2001 Aug;23(8):725-32. doi: 10.1002/bies.1102. Bioessays. 2001. PMID: 11494321 Review. - Ribosome dynamics: insights from atomic structure modeling into cryo-electron microscopy maps.
Mitra K, Frank J. Mitra K, et al. Annu Rev Biophys Biomol Struct. 2006;35:299-317. doi: 10.1146/annurev.biophys.35.040405.101950. Annu Rev Biophys Biomol Struct. 2006. PMID: 16689638 Review.
Cited by
- Case Report: Bayesian Statistical Inference of Experimental Parameters via Biomolecular Simulations: Atomic Force Microscopy.
Fuchigami S, Niina T, Takada S. Fuchigami S, et al. Front Mol Biosci. 2021 Mar 10;8:636940. doi: 10.3389/fmolb.2021.636940. eCollection 2021. Front Mol Biosci. 2021. PMID: 33778008 Free PMC article. - Cryo-EM reveals the conformational epitope of human monoclonal antibody PAM1.4 broadly reacting with polymorphic malarial protein VAR2CSA.
Raghavan SSR, Dagil R, Lopez-Perez M, Conrad J, Bassi MR, Quintana MDP, Choudhary S, Gustavsson T, Wang Y, Gourdon P, Ofori MF, Christensen SB, Minja DTR, Schmiegelow C, Nielsen MA, Barfod L, Hviid L, Salanti A, Lavstsen T, Wang K. Raghavan SSR, et al. PLoS Pathog. 2022 Nov 16;18(11):e1010924. doi: 10.1371/journal.ppat.1010924. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36383559 Free PMC article. - Cross-validation in cryo-EM-based structural modeling.
Falkner B, Schröder GF. Falkner B, et al. Proc Natl Acad Sci U S A. 2013 May 28;110(22):8930-5. doi: 10.1073/pnas.1119041110. Epub 2013 May 14. Proc Natl Acad Sci U S A. 2013. PMID: 23674685 Free PMC article. - Single-molecule nanometry for biological physics.
Kim H, Ha T. Kim H, et al. Rep Prog Phys. 2013 Jan;76(1):016601. doi: 10.1088/0034-4885/76/1/016601. Epub 2012 Dec 18. Rep Prog Phys. 2013. PMID: 23249673 Free PMC article. Review. - Ring closure activates yeast γTuRC for species-specific microtubule nucleation.
Kollman JM, Greenberg CH, Li S, Moritz M, Zelter A, Fong KK, Fernandez JJ, Sali A, Kilmartin J, Davis TN, Agard DA. Kollman JM, et al. Nat Struct Mol Biol. 2015 Feb;22(2):132-7. doi: 10.1038/nsmb.2953. Epub 2015 Jan 19. Nat Struct Mol Biol. 2015. PMID: 25599398 Free PMC article.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. - PubMed
- Alberts B. The cell as a collection of protein machines - preparing the next generation of biologists. Cell. 1998;92:291–294. - PubMed
- Arkhipov A, Freddolino PL, Schulten K. Stability and dynamics of virus capsids described by coarse-grained modeling. Structure. 2006;14:1767–1777. - PubMed
- Brünger AT, Brooks CL, III, Karplus M. Stochastic boundary conditions for molecular dynamics simulations of ST2 water. Chem. Phys. Lett. 1984;105:495–498.
- Chapman MS. Restrained real-space macromolecular atomic refinement using a new resolution-dependent electron-density function. Acta Cryst. A. 1995;51:69–80.
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR005969/RR/NCRR NIH HHS/United States
- P41-RR01219/RR/NCRR NIH HHS/United States
- P41 RR001219/RR/NCRR NIH HHS/United States
- R01 GM055440/GM/NIGMS NIH HHS/United States
- R37-GM29169/GM/NIGMS NIH HHS/United States
- P41 RR005969-18/RR/NCRR NIH HHS/United States
- P41-RR05969/RR/NCRR NIH HHS/United States
- R01-GM55440/GM/NIGMS NIH HHS/United States
- R37 GM029169/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources