A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating - PubMed (original) (raw)
A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating
Benjamin R Myers et al. Neuron. 2008.
Abstract
TRP cation channels function as cellular sensors in uni- and multicellular eukaryotes. Despite intensive study, the mechanisms of TRP channel activation by chemical or physical stimuli remain poorly understood. To identify amino acid residues crucial for TRP channel gating, we developed an unbiased, high-throughput genetic screen in yeast that uncovered rare, constitutively active mutants of the capsaicin receptor, TRPV1. We show that mutations within the pore helix domain dramatically increase basal channel activity and responsiveness to chemical and thermal stimuli. Mutation of corresponding residues within two related TRPV channels leads to comparable effects on their activation properties. Our data suggest that conformational changes in the outer pore region are critical for determining the balance between open and closed states, providing evidence for a general role for this domain in TRP channel activation.
Figures
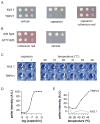
Figure 1. Mammalian TRPV1 forms functional channels in yeast
(A) Serial dilution assay for growth of yeast strains transformed with plasmids encoding mouse Kir2.1 or rat TRPV1. Yeast were resuspended to approximately the same density, spotted (from left to right) on indicated media and allowed to grow at 30 °C for 3 days. (B) Serial dilution assay for wild type TRPV1 versus TRPV1 Δ777–820, grown as described in (A). (C) Representative cobalt uptake assay performed with yeast expressing TRPV1 or Kir2.1. Cells were exposed to 10 μM capsaicin (at 30°C) or elevated bath temperature (36–48°C) and cobalt accumulation visualized in cell pellets collected in microtiter wells. (D) Intensity of cobalt sulfide staining was determined using pixel quantitation (arbitrary units), yielding a capsaicin concentration response relationship that could be fit with a sigmoid function. (E) Quantitation of TRPV1 (black) or Kir2.1 (green) temperature responsiveness in yeast using the cobalt uptake assay, revealing a TRPV1 thermal activation threshold near 40°C.
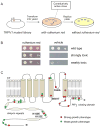
Figure 2. Yeast genetic screen identifies gain-of-function TRPV1 alleles
(A) Outline of the screening procedure: yeast were transformed with the randomly mutagenized TRPV1 library and grown for 2 days following replica plating. Subsequently, toxic alleles were identified and picked from the ruthenium red plate for secondary analysis and plasmid rescue. (B) Serial dilution assays of cultures expressing wild type TRPV1 or representative mutants illustrating the range of toxic phenotypes recovered from the yeast screen. (C) Each allele recovered in the screen was mapped onto a topology diagram of TRPV1. Red or green spheres indicate location of mutations causing strong or weak toxic phenotypes, respectively. N-terminal ankyrin repeats, transmembrane helices (S1–S6), and putative C-terminal PIP2 binding domain are shown. Mutants exhibiting high constitutive activity in electrophysiological assays (see figure 3) are labeled for reference.
Figure 3. Mutants recovered from the yeast screen display altered electrophysiological properties
Representative current traces from two-electrode voltage clamp (TEVC, +80 mV) recordings of oocytes expressing wild type TRPV1 (A), potentiated mutants (B), or constitutive mutants (C). Oocytes were challenged with protons (pH 6.4), capsaicin (10 μM), or ruthenium red (10 μM). (D) Quantitation of basal currents for all TRPV1 mutants exhibiting constitutive activity (normalized to a saturating capsaicin response; mean ± s.e.m., n ≥ 3 per construct). Currents in the presence of ruthenium red (10 μM) were used as baseline. For wild type TRPV1, >90% block of capsaicin-evoked currents was achieved after 30 seconds of ruthenium red treatment, and the wild-type value of Ibasal/Icap was subtracted from all measurements. (E) Quantitation of pH 6.4 responses (normalized to a saturating capsaicin response) for all TRPV1 mutants exhibiting constitutive or potentiated activity. Data represent mean ± s.e.m., n ≥ 3 per construct.
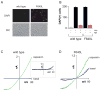
Figure 4. F640L mutant TRPV1 channels are toxic and display high basal activity in mammalian cells
(A) Representative image of HEK293 cells transfected with wild type TRPV1 or F640L mutant after staining with 4′,6-Diamidino-2-Phenylindole (DAPI) to identify dead cells. Corresponding differential interference contrast (DIC) images are shown below. (B) Quantitation of cell death assay as performed in (A). Black and red bars represent cell death in the absence or presence of ruthenium red (3 μM, RR), respectively (mean ± s.e.m.). Death among cells transfected with wild type TRPV1 with or without capsaicin (1 μM, cap) is shown for comparison. Background cell death was determined from cells transfected with vector alone and subtracted from each measurement. (C, D) Representative inside-out patch recording from HEK293 cells transfected with wild type TRPV1 or the F640L mutant. Voltage ramps under basal conditions (blue) or in the presence of 10 μM capsaicin (green) are shown. Inset: for the wild type channel, 25 consecutive ramp traces were averaged and leak-subtracted to accurately derive the ensemble average basal current.
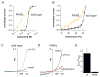
Figure 5. F640L TRPV1 mutant channels show enhanced chemical and thermal sensitivity, but decreased proton potentiation
(A) TEVC recording of TRPV1 wild type (black) or F640L (orange) channels reveals leftward shift in the agonist dose-response relationship (mean ± s.e.m. at +80 mV, n = 4 oocytes per condition). (B) Inside-out patch recording from HEK293 cells transfected with wild type (black) or F640L (orange) channels stimulated with a temperature ramp from 10 to 44 °C (mean ± s.e.m. at −60 mV, n ≥ 4 patches per condition) reveals hypersensitivity to heat. (C, D) Voltage ramp traces from whole-cell patch clamp recordings of HEK293 cells expressing wild type or F640L mutant channels. Blue traces indicate basal channel current at room temperature in pH 7.4 bath solution, while green traces indicate channel current after perfusion with pH 6.2 bath solution. Subsequent addition of capsazepine (30 μM, red) efficiently inhibited F640L basal current. (E) Quantitation of fold potentiation (at +80 mV) at pH 6.2 vs. 7.4 for wild type and F640L channels, as in (C) and (D) (mean ± s.e.m., n = 4 – 6 cells per condition).
Figure 6. Additional substitutions in the TRPV1 pore helix affect channel activation
(A) Alignment of the pore helices and selectivity filters from various TRPV channels (r = rat, h = human, c = chicken). Helicity index for rat TRPV1 (0–10, generated using PSIPRED) is shown above alignment. (B) Summary of F640 saturation screen. Transformants harboring a library of TRPV1 mutants (with a randomized F640 codon) were scored according to growth pattern and mutant plasmids sequenced to determine the amino acid substitution. Note that F640A and F640T were scored as both “wild type” and “weak loss of function”, consistent with an intermediate phenotype. (C, D) Quantitation of normalized basal or pH-evoked currents for mutants recovered from the pore helix screen, analyzed by TEVC as in figure 3. *p ≤ 0.01, Student’s t-test.
Figure 7. The gating function of the pore helix is conserved across TRPV channels
Voltage ramp traces from representative inside-out macropatches excised from oocytes expressing wild type human TRPV3 (A) vs. T636S mutant (B). Patches were allowed to stabilize for five minutes after excision. Blue traces indicate basal current at room temperature. Inset represents ensemble average basal current for wild type TRPV3. Red trace shows current at 10°C, illustrating block of T636S basal activity by cold temperature. Corresponding current vs. time plots are shown below each set of voltage ramps, illustrating that wild type and mutant channels respond similarly to 300 μM 2-APB. (C) Quantitation of basal current (normalized to 300 μM 2-APB response) for TRPV3 wild type or T636S mutant channels (n ≥ 4 patches per condition). (D) TRPV3 T636S mutant caused massive toxicity in transfected HEK293 cells that was blocked by ruthenium red (RR, 3 μM, red bar). Wild type TRPV3 is shown for comparison. (E) Representative traces from TEVC recordings of oocytes expressing wild type rat TRPV2 vs. F603L mutant. Oocytes were exposed to 1 or 3 mM 2-APB (green or yellow bars, respectively). (F) Quantitation of 1 mM 2-APB evoked responses in cells expressing wild type TRPV2 or F603L mutant channel (normalized to the 3 mM 2-APB response) reveals that the F603L mutant displays enhanced sensitivity to a lower concentration of 2-APB (p ≤ 0.001, Student’s t-test).
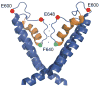
Figure 8. A structural model for the pore region of TRP channels
A model of the S5-pore-S6 region of TRPV1 as inspired by the Kv1.2 structure (PDB: 2R9R), prepared with PyMOL software (Delano, 2002). The pore helix is shown in orange. Relative location of the F640 residue is shown in green, and E600 and E648 residues previously implicated in proton modulation are shown in red.
Similar articles
- Toward elucidating the heat activation mechanism of the TRPV1 channel gating by molecular dynamics simulation.
Wen H, Qin F, Zheng W. Wen H, et al. Proteins. 2016 Dec;84(12):1938-1949. doi: 10.1002/prot.25177. Epub 2016 Oct 24. Proteins. 2016. PMID: 27699868 Free PMC article. - Contribution of the putative inner-pore region to the gating of the transient receptor potential vanilloid subtype 1 channel (TRPV1).
Susankova K, Ettrich R, Vyklicky L, Teisinger J, Vlachova V. Susankova K, et al. J Neurosci. 2007 Jul 11;27(28):7578-85. doi: 10.1523/JNEUROSCI.1956-07.2007. J Neurosci. 2007. PMID: 17626219 Free PMC article. - Role of the outer pore domain in transient receptor potential vanilloid 1 dynamic permeability to large cations.
Munns CH, Chung MK, Sanchez YE, Amzel LM, Caterina MJ. Munns CH, et al. J Biol Chem. 2015 Feb 27;290(9):5707-24. doi: 10.1074/jbc.M114.597435. Epub 2015 Jan 7. J Biol Chem. 2015. PMID: 25568328 Free PMC article. - ThermoTRP channels as modular proteins with allosteric gating.
Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. Latorre R, et al. Cell Calcium. 2007 Oct-Nov;42(4-5):427-38. doi: 10.1016/j.ceca.2007.04.004. Epub 2007 May 17. Cell Calcium. 2007. PMID: 17499848 Review.
Cited by
- Mapping temperature-dependent conformational change in the voltage-sensing domain of an engineered heat-activated K+ channel.
Chen H, Deng J, Cui Q, Chanda B, Henzler-Wildman K. Chen H, et al. Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2017280118. doi: 10.1073/pnas.2017280118. Proc Natl Acad Sci U S A. 2021. PMID: 33782120 Free PMC article. - Hot target on nociceptors: perspectives, caveats and unique features.
Szolcsányi J. Szolcsányi J. Br J Pharmacol. 2008 Dec;155(8):1142-4. doi: 10.1038/bjp.2008.374. Epub 2008 Nov 10. Br J Pharmacol. 2008. PMID: 18997812 Free PMC article. - Functional and Modeling Studies of the Transmembrane Region of the TRPM8 Channel.
Bidaux G, Sgobba M, Lemonnier L, Borowiec AS, Noyer L, Jovanovic S, Zholos AV, Haider S. Bidaux G, et al. Biophys J. 2015 Nov 3;109(9):1840-51. doi: 10.1016/j.bpj.2015.09.027. Biophys J. 2015. PMID: 26536261 Free PMC article. - Heat activation is intrinsic to the pore domain of TRPV1.
Zhang F, Jara-Oseguera A, Chang TH, Bae C, Hanson SM, Swartz KJ. Zhang F, et al. Proc Natl Acad Sci U S A. 2018 Jan 9;115(2):E317-E324. doi: 10.1073/pnas.1717192115. Epub 2017 Dec 26. Proc Natl Acad Sci U S A. 2018. PMID: 29279388 Free PMC article. - Temperature sensitive contact modes allosterically gate TRPV3.
Burns D, Venditti V, Potoyan DA. Burns D, et al. PLoS Comput Biol. 2023 Oct 13;19(10):e1011545. doi: 10.1371/journal.pcbi.1011545. eCollection 2023 Oct. PLoS Comput Biol. 2023. PMID: 37831724 Free PMC article.
References
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, Sakata T. Association of a mutation in TRPV3 with defective hair growth in rodents. The Journal of investigative dermatology. 2006;126:2664–2672. - PubMed
- Bandell M, Dubin AE, Petrus MJ, Orth A, Mathur J, Hwang SW, Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nature neuroscience. 2006;9:493–500. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 NS047723-16/NS/NINDS NIH HHS/United States
- R37 NS047723-15/NS/NINDS NIH HHS/United States
- R37 NS047723-13/NS/NINDS NIH HHS/United States
- R37 NS047723/NS/NINDS NIH HHS/United States
- R37 NS047723-14/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical