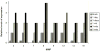Multiple expression of matrix metalloproteinases in murine neurocysticercosis: Implications for leukocyte migration through multiple central nervous system barriers - PubMed (original) (raw)
Multiple expression of matrix metalloproteinases in murine neurocysticercosis: Implications for leukocyte migration through multiple central nervous system barriers
Jorge I Alvarez et al. Brain Res. 2008.
Abstract
During the course of murine neurocysticercosis (NCC), disruption of the unique protective barriers in the central nervous system (CNS) is evidenced by extravasation of leukocytes. This process varies according to the anatomical sites and diverse vascular beds analyzed. To examine mechanisms involved in the observed differences, the expression and activity of eight matrix metalloproteinases (MMPs) were analyzed in a murine model of NCC. The mRNA expression of the MMPs studied was upregulated as a result of infection, and active MMPs were mainly detected in leukocytes migrating into the brain. Polarized expression and gelatinolytic activity of several MMPs were identified in immune cells extravasating pial vessels as early as 1 day post infection. In contrast, leukocytes expressing active MMPs and extravasating parenchymal vessels were not observed until 5 weeks post infection. In ventricular areas, most of the MMP activity was detected in leukocytes traversing the ependyma from leptomeningeal infiltrates. In addition, immune cells continued to express active MMPs after exiting vessels suggesting that enzymatic activity of MMPs is not just required for diapedesis. These results correlate with our previous studies showing differential kinetics in the disruption of the CNS barriers upon infection and help document the important role of MMPs during leukocyte infiltration and inflammation.
Figures
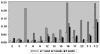
Figure 1. MMP expression is upregulated during the course of murine NCC
Total RNA was isolated from mock and infected controls at different post infection times. After reverse transcription in presence of α-33P-dCTP, the cDNA probes were hybridized to GEArray Q series membranes. The levels of mRNA expression of distinct MMPs were calculated in arbitrary units and normalized to the average of housekeeping genes. Normalized values are shown in the y-axis and represent the mean of two independent experiments. MMPs are denoted by their number. T-1 and T-2 represent TIMP-1 and TIMP-2 respectively.
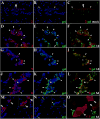
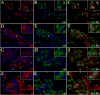
Figure 2. Extravasation of leukocytes through pial vessels involves expression of active MMPs early in the infectious process
ISZ and IF were performed in mock and animals infected with M. corti for 1 (1d) and 3 days (3d). MMPs are labeled red (Rhodamine red X) and their activity, detected as gelatin (gel) degradation appears green (fluorescein). Nuclear staining (DAPI) is blue. Three images (MMP+DAPI)-(gel+DAPI)-(MMP+gel) of the same field were included for clarity. (A) Low expression of MMP-2 in pial vessel (large arrowhead) of mock infected animal. 40X (B) Active gelatinolysis in leukocytes (arrowheads) located in vicinity of pial vessel (C) Modest expression of active MMP-2 in leukocytes (small arrowheads) extravasating pial vessel (large arrowhead) (D) MMP-2 expression in pial vessel (asterisk) and infiltrating cells (arrowheads). 1d - 100X (E) Gelatinolytic activity in cells (arrowheads) traversing pial vessel (asterisk) (F) Gelatinolysis and MMP-2 expression in cells (arrowheads) extravasating pial vessel (asterisk) and accumulating in perivascular areas (G) Extravasating cells (arrowheads) showing polarized MMP-9 expression from the lumen towards the basal lamina in a pial vessel (asterisk). 1d – 100X (H) Polarized gelatinolysis in cells (arrowheads) traversing pial vessel (asterisk) (I) Active MMP-9 expression in cells (arrowheads) infiltrating into the CNS through a pial vessel (asterisk) (J) Extravasating cells (arrowheads) displaying polarized MMP-8 expression from the lumen towards the basal lamina in pial vessel (asterisk). 3d – 100X (K) Polarized gelatinolysis in cells (arrowheads) traversing pial vessel (asterisk) (L) Active MMP-8 expression in cells (arrowheads) traversing a pial vessel (asterisk) (M) Expression of MMP-7 in pial vessels (arrowheads). 1d - 63X (N) Infiltrating leukocytes (arrowheads) displaying gelatinolysis (O) High power view of area marked in M and N, showing lack of gelatinolysis in MMP-7 positive pial vessels (large arrowheads), and high expression in basal lamina. Gelatin degradation is seen in MMP-7 negative leukocytes (small arrowheads).
Figure 3. Kinetics and relative levels of MMP expression in leukocytes extravasating and infiltrating the CNS during the course of murine NCC
Mice were intracranially inoculated with M. corti and sacrificed at the indicated times. 3 animals were analyzed per each infection time. MMPs (x axis) were determined using the polyclonal antibodies described in material and methods. The relative number of cells at each post infection time was arbitrarily assigned a number from 0 to 4 representing absent to abundant.
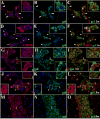
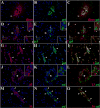
Figure 4. Expression of active MMPs detected by gelatin cleavage in leptomeningeal infiltrates during the course of murine NCC
ISZ detected as gel (green) degradation and MMP expression (red) were performed in M. corti infected animals 1 (1w), 3 (3w) and 5 weeks (5w) post-infection. Nuclear staining (DAPI) is blue. Three images (MMP+DAPI)-(gel+DAPI)-(MMP+gel) of the same field were included for clarity. High power view insets (2.5X times magnified) of pictures A to L were added to better illustrate MMP activity. (A) MMP-2 expression in leukocytes extravasating pial vessels (asterisks) and infiltrating leptomeninges (large arrowheads). Astrocyte endfeet processes (small arrowheads) also express MMP-2. 1w - 40X (B) Same field as A showing gelatinolysis (C) Cells extravasating pial vessels (asterisks) and infiltrating leptomeninges (large arrowheads) express active MMP-2. Astrocyte endfeet (small arrowheads) also express MMP-2, but lack gelatinolysis (D) CD11b+ leukocytes extravasating pial vessels (asterisks) and accumulating in internal leptomeninges (arrowheads). 1w - 40X (E) same field as D showing gelatin degradation (F) CD11b+ infiltrates (arrowheads) actively degrading gelatin (G) MMP-3 expression in leukocytes infiltrating internal leptomeninges (inset 1) and pial vessels (inset 2). 3w - 40X (H) same field as G showing gelatin degradation (I) Infiltrates (inset 1) in internal leptomeninges express active MMP-3, gelatin degradation is also detected in pial vessels (inset 2) (J) Infiltrating leukocytes (inset 1) and pial vessels (asterisks - inset 2) in internal leptomeninges expressing MMP-7. 3w - 40X (K) same field as J showing gelatin degradation (L) Active MMP-7 is moderately expressed in infiltrating cells (inset 1). Low gelatin degradation is detected in pial vessels (asterisks - inset 2) (M) High expression of MMP-8 in leptomeningeal infiltrate (i). Astrocyte endfeet (small arrowhead) opposed to the pia also express MMP-8. 5w - 40X (N) same field as M showing gelatinolysis (O) Active expression of MMP-8 in leptomeningeal infiltrate (i). Astrocyte endfeet lack gelatin degradation.
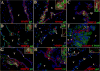
Figure 5. Active MMPs detected by collagen cleavage are present in inflammatory infiltrates of mice infected with M. corti
ISZ using collagen (col)-oregon 488 (green) as substrate and MMP expression (red) were performed in M. corti infected animals 1w, 3w and 5w post-infection. Nuclear staining (DAPI) is blue. Three images (MMP+DAPI)-(col+DAPI)-(MMP+col) of the same field were included for clarity. High power view insets (2.5X times magnified) were added to better illustrate MMP activity (A) MMP-12 expressed by leukocytes infiltrating (inset) external leptomeninges and by astrocyte endfeet (arrowheads). 1w - 40X (B) Same field as A, but showing collagenolysis (C) Collagen cleavage is detected in MMP-12 expressing leukocytes (inset), but no in astrocyte endfeet (arrowheads) (D) Leukocytes in inflammatory infiltrates (i) at internal leptomeninges expressing MMP16. 3w - 40X (E) Same field as D showing collagenolysis (F) Leukocytes (i) infiltrating internal leptomeninges express active MMP-16 (G) MMP-13 expression in internal leptomeninges infiltrates (inset) and astrocyte endfeet (arrowheads). 5w - 40X (H) same field as G, but showing collagen cleavage (I) Active MMP-13 is modestly expressed by some cells in inflammatory infiltrates (inset), inactive MMP-13 is detected in astrocyte endfeet (arrowheads) (J) High expression of MMP-8 in internal leptomeninges infiltrates (inset) and astrocyte endfeet (arrowheads). 5w - 40X (K) Same field as J, showing collagenolysis (L) Active expression of MMP-8 in leptomeningeal infiltrates (inset), inactive enzyme is detected in few inflammatory cells and astrocyte endfeet (arrowheads).
Figure 6. Expression of active MMPs in leukocytes infiltrating CNS parenchyma
ISZ using gel and col(green) combine with IF detection of MMPs (red) were used to determine the expression of active MMPs in leukocytes extravasating parenchymal vessels 5w post-infection. Nuclear staining is blue. Three images (MMP+DAPI)-(gel+DAPI)-(MMP+gel) of the same field were included for clarity. High power view insets (2.5X times magnified) of pictures D to F and J to L were added to better illustrate MMP activity (A) Fibronectin extravasation (arrowhead) in parenchymal vessel. 40X (B) same field as A showing gelatin degradation (C) Active gelatinolysis in parenchymal vessel displaying fibronectin extravasation (D) MMP-2 expression in leukocytes extravasating parenchymal vessel (large arrowhead - inset 1), microglia-astrocyte like cells (inset 2) and astrocyte endfeet (small arrowheads). 40X (E) same field as D showing gelatin degradation (F) Active MMP-2 expression in some of the leukocytes extravasating a parenchymal vessel (large arrowhead-inset 1) and in microglia-astrocyte like cells (inset 2). MMP-2 expressed in astrocyte endfeet (small arrowheads) does not cleave gelatin (G) Expression of MMP-8 in leukocytes extravasating parenchymal vessels (arrowheads). 40X (H) same field as G showing gelatin degradation (I) Active MMP-8 expression by leukocytes in perivascular infiltrates of parenchymal vessels (arrowheads) (J) Leukocytes extravasating parenchymal vessel (asterisk) displayed moderate MMP-13 expression (inset). 40X (K) Same field as J showing collagen degradation (L) Active collagen degradation is detected in few MMP-13 positive leukocytes (inset) extravasating a parenchymal vessel (asterisk) (M) MMP-16 expression in leukocytes (arrowheads) extravasating a parenchymal vessel. 40X (N) same field as M showing collagenolysis (O) Active expression of MMP-16 in leukocytes extravasating a parenchymal vessel.
Figure 7. MMP expression in ependyma, CP and leukocytes infiltrating ventricle in mice infected with M. corti
ISZ with gel and col (green) as substrates and IF with polyclonal antibodies against distinct MMPs (red) were used to determine the expression of active MMPs in ventricular areas during infection. Panel D shows cadherin expression (red) and CD11b+ cells (green). High power view insets (2.5X times magnified) were included in some pictures to better illustrate MMP activity. Nuclear staining is blue. Ventricle (v), ependyma (e) and choroid plexus (cp). (A) MMP-8 expression and low gelatinolysis is detected in e of mock infected animal. 40X (B) MMP-8 expression increased in e. Active and polarized expression of this enzyme was found in leukocytes (inset) traversing this structure. 1w 40X (C) MMP-12 expression was detected in cp and leukocytes (inset) migrating into the v, active enzyme was only detected in leukocytes (inset). 1w 40X (D) Active MMP-16 expressed in leukocytes (inset) migrating through e into v. 1w - 40X (E) Expression of active MMP-9 in cells (large arrowhead – inset 1) moving from subependyma into ventricles. Subependymal astrocyte endfeet (small arrowheads – inset 2) also express MMP-9 able to degrade gelatin. 1w - 40X (F) High infiltration of CD11b+ cells into ventricle through holes (arrowheads) in the e labeled with pan-cadherin antibody. 3w - 63X (G) Active gelatinolysis by MMP-12 was detected in leukocytes (arrowheads - inset) located under e. Moderate upregulation of inactive MMP-12 was detected in e. 3w - 40X (H) Active MMP-8 expression in leukocytes (arrowhead – inset) located under e. Moderate upregulation of inactive MMP-8 is detected in e. 3w - 40X (I) Subependymal astrocyte endfeet (large arrowheads - inset) expressed MMP-2 displaying gelatinolytic activity. No MMP-2 expression was detected in cp or in cells extravasating the cp (small arrowheads). 3w - 40X
Similar articles
- Differential changes in junctional complex proteins suggest the ependymal lining as the main source of leukocyte infiltration into ventricles in murine neurocysticercosis.
Alvarez JI, Teale JM. Alvarez JI, et al. J Neuroimmunol. 2007 Jul;187(1-2):102-13. doi: 10.1016/j.jneuroim.2007.05.005. Epub 2007 Jun 26. J Neuroimmunol. 2007. PMID: 17597230 Free PMC article. - Evidence for differential changes of junctional complex proteins in murine neurocysticercosis dependent upon CNS vasculature.
Alvarez JI, Teale JM. Alvarez JI, et al. Brain Res. 2007 Sep 12;1169:98-111. doi: 10.1016/j.brainres.2007.07.010. Epub 2007 Jul 14. Brain Res. 2007. PMID: 17686468 Free PMC article. - An Update on the Role of Matrix Metalloproteinases in the Pathogenesis of Multiple Sclerosis.
Boziki M, Grigoriadis N. Boziki M, et al. Med Chem. 2018 Feb 6;14(2):155-169. doi: 10.2174/1573406413666170906122803. Med Chem. 2018. PMID: 28875862 Review. - Mesocestoides corti intracranial infection as a murine model for neurocysticercosis.
Alvarez JI, Mishra BB, Gundra UM, Mishra PK, Teale JM. Alvarez JI, et al. Parasitology. 2010 Mar;137(3):359-72. doi: 10.1017/S0031182009991971. Epub 2010 Jan 29. Parasitology. 2010. PMID: 20109250 Review.
Cited by
- Targeting innate immunity to protect and cure Alzheimer's disease: opportunities and pitfalls.
Cisbani G, Rivest S. Cisbani G, et al. Mol Psychiatry. 2021 Oct;26(10):5504-5515. doi: 10.1038/s41380-021-01083-4. Epub 2021 Apr 14. Mol Psychiatry. 2021. PMID: 33854189 Review. - Taenia crassiceps injection into the subarachnoid space of rats simulates radiological and morphological features of racemose neurocysticercosis.
Hamamoto Filho PT, Fabro AT, Rodrigues MV, Bazan R, Vulcano LC, Biondi GF, Zanini MA. Hamamoto Filho PT, et al. Childs Nerv Syst. 2017 Jan;33(1):119-123. doi: 10.1007/s00381-016-3239-3. Epub 2016 Sep 9. Childs Nerv Syst. 2017. PMID: 27613638 - Increased accumulation of regulatory granulocytic myeloid cells in mannose receptor C type 1-deficient mice correlates with protection in a mouse model of neurocysticercosis.
Mishra PK, Morris EG, Garcia JA, Cardona AE, Teale JM. Mishra PK, et al. Infect Immun. 2013 Apr;81(4):1052-63. doi: 10.1128/IAI.01176-12. Epub 2013 Jan 14. Infect Immun. 2013. PMID: 23319563 Free PMC article. - Meningeal B Cell Clusters Correlate with Submeningeal Pathology in a Natural Model of Multiple Sclerosis.
Church ME, Ceja G, McGeehan M, Miller MC, Farias P, Sánchez MD, Swain GP, Assenmacher CA, Stopa EG, Vite CH, Bar-Or A, Alvarez JI. Church ME, et al. J Immunol. 2021 Jul 1;207(1):44-54. doi: 10.4049/jimmunol.2000514. Epub 2021 Jun 23. J Immunol. 2021. PMID: 34162727 Free PMC article. - Transcriptome analysis of the ependymal barrier during murine neurocysticercosis.
Mishra PK, Teale JM. Mishra PK, et al. J Neuroinflammation. 2012 Jun 25;9:141. doi: 10.1186/1742-2094-9-141. J Neuroinflammation. 2012. PMID: 22731103 Free PMC article.
References
- Alvarez JI, Teale JM. Breakdown of the blood brain barrier and blood-cerebrospinal fluid barrier is associated with differential leukocyte migration in distinct compartments of the CNS during the course of murine NCC. J Neuroimmunol. 2006;173:45–55. - PubMed
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS035974/NS/NINDS NIH HHS/United States
- NS35974/NS/NINDS NIH HHS/United States
- AI 59703/AI/NIAID NIH HHS/United States
- R01 AI059703/AI/NIAID NIH HHS/United States
- P01 AI057986/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources