Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery - PubMed (original) (raw)
Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery
Rong Zhang et al. Int J Radiat Biol. 2008 Jun.
Abstract
Purpose: To characterize structural and functional injuries following a single dose of whole-thorax irradiation that might be survivable after a nuclear attack/accident.
Methods: Rats were exposed to 5 or 10 Gy of X-rays to the whole thorax with other organs shielded. Non-invasive measurements of breathing rate and arterial oxygen saturation, and invasive evaluations of bronchoalveolar lavage fluid, (for total protein, Clara cell secretory protein), vascular reactivity and histology were conducted for at least 6 time points up to 52 weeks after irradiation.
Results: Irradiation with 10 Gy resulted in increased breathing rate, a reduction in oxygen saturation, an increase in bronchoalveolar lavage fluid protein and attenuation of vascular reactivity between 4-12 weeks after irradiation. These changes were not observed with the lower dose of 5 Gy. Histological examination revealed perivascular edema at 4-8 weeks after exposure to both doses, and mild fibrosis beyond 20 weeks after 10 Gy.
Conclusions: Single-dose exposure of rat thorax to 10 but not 5 Gy X-irradiation resulted in a decrease in oxygen uptake and vasoreactivity and an increase in respiratory rate, which paralleled early pulmonary vascular pathology. Vascular edema resolved and was replaced by mild fibrosis beyond 20 weeks after exposure, while lung function recovered.
Figures
Figure 1
Hematoxylin-eosin-stained lung sections from a minimum of 7 rats were examined at each time point. Representative images for the major findings are shown as follows: a) control (0 Gy) at 3 days after irradiation; b) 0 Gy at 52 wks after irradiation; c) 5 Gy at 8 wks after irradiation, vessel wall hyalinization marked with arrow; d) 5 Gy at 20 wks after irradiation, chronic vessel changes marked with arrow; e) 10 Gy at 4 wks after irradiation, perivascular edema marked with arrow; f) 10 Gy at 52 wks after irradiation, mononuclear cells marked with arrow.
Figure 2
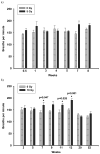
The breathing rate of rats was measured non-invasively using an airtight plethysmograph. Breaths per min following 5 Gy (panel a) and 10 Gy (panel b) were compared to controls. Values are mean breaths per min ± sem; _n_=4–6 (5 Gy), _n_=6–15 (10 Gy). No increase in breathing rate was observed following exposure to 5 Gy up to 8 wks. A significant elevation (_p_≤0.05 vs Ctrl) in breathing rate was noted at 7, 11 and 12 wks following irradiation with 10 Gy.
Figure 3
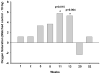
Oxygen saturation was measured non-invasively using a veterinary pulse oximeter 10 min after exposure to a hypoxic gas mixture of 12% oxygen in nitrogen. Rats were compared to controls at each time point. Data is shown for exposure to 10 Gy. The difference of mean oxygen saturation values between the age-matched controls and irradiated rats is presented for 10 Gy only. The mean ± sem values recorded were: wk1–10 Gy: 83.0 ±1.6, 0 Gy: 84.2 ± 0.7; wk2–10 Gy: 82.8 ± 1.0, 0 Gy: 84.0 ± 1.0; wk5–10 Gy: 80.3 ± 1.3, 0 Gy: 83.7 ± 1.4; wk8–10 Gy: 81.3 ± 1.5, 0 Gy: 85.0 ± 1.8; wk11–10 Gy: 79.5 ± 1.2, 0 Gy: 85.3 ± 1.5; wk13–10 Gy: 80.8 ± 0.9, 0 Gy: 86.1 ± 1.6; wk20–10 Gy: 85.7 ± 2.4, 0 Gy: 83.3 ± 1.2; wk52–10 Gy: 85.2 ± 1.4, 0 Gy: 86.3 ± 1.3; _n_=6–15. A significant reduction (_p_≤0.05 vs Ctrl) in oxygen saturation was observed in rats exposed to a dose of 10 Gy at 11 and 13 wks after irradiation.
Figure 4
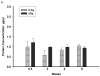
Total protein levels in the BALF of rats exposed to a dose of 5 Gy (panel a) and 10 Gy (panel b) compared to controls. Each data point represents mean ± sem; _n_=3–8. Following exposure to 5 Gy, no significant increase in total proteins was observed up to 8 wks after irradiation. Total Proteins were significantly elevated (_p_≤0.05 vs Ctrl) at 4 wks and at 8 wks after irradiation in rats exposed to a dose of 10 Gy compared to un-irradiated control rats.
Figure 4
Total protein levels in the BALF of rats exposed to a dose of 5 Gy (panel a) and 10 Gy (panel b) compared to controls. Each data point represents mean ± sem; _n_=3–8. Following exposure to 5 Gy, no significant increase in total proteins was observed up to 8 wks after irradiation. Total Proteins were significantly elevated (_p_≤0.05 vs Ctrl) at 4 wks and at 8 wks after irradiation in rats exposed to a dose of 10 Gy compared to un-irradiated control rats.
Figure 5
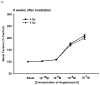
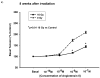
Representative graphs showing vascular reactivity to Ang II in pulmonary artery rings with a dose of 5 Gy at different Ang II concentrations (panel a) and time points (panel b). Each data point represents mean ± sem and is expressed as % of baseline contraction; _n_=32 rings from 8 rats (panel a), _n_=20–34 rings from 5–9 rats (panel b). No significant differences were observed at 8 wks after irradiation (panel a). The response of pulmonary artery rings to 10−7 M Ang II at the different times after irradiation found no significant differences between the two groups (panel b).
Figure 5
Representative graphs showing vascular reactivity to Ang II in pulmonary artery rings with a dose of 5 Gy at different Ang II concentrations (panel a) and time points (panel b). Each data point represents mean ± sem and is expressed as % of baseline contraction; _n_=32 rings from 8 rats (panel a), _n_=20–34 rings from 5–9 rats (panel b). No significant differences were observed at 8 wks after irradiation (panel a). The response of pulmonary artery rings to 10−7 M Ang II at the different times after irradiation found no significant differences between the two groups (panel b).
Figure 6
Representative graphs showing vascular reactivity to Ang II in pulmonary artery rings with a dose of 10 Gy at different Ang II concentrations (panel a) and time points (panel b). Each data point represents mean ± sem and is expressed as % of baseline contraction; _n_=27 rings from 7 rats (panel a), _n_=16–40 rings from 4–11 rats (panel b). There was a fall in reactivity in irradiated vessels at 8 wks after irradiation (panel a). The response of the rings to 10−7 M Ang II at different time points following exposure showed significant attenuation to vasoreactivity at 4, 8 and 52 wks after irradiation (* p<0.01 vs Ctrl, panel b).
Figure 6
Representative graphs showing vascular reactivity to Ang II in pulmonary artery rings with a dose of 10 Gy at different Ang II concentrations (panel a) and time points (panel b). Each data point represents mean ± sem and is expressed as % of baseline contraction; _n_=27 rings from 7 rats (panel a), _n_=16–40 rings from 4–11 rats (panel b). There was a fall in reactivity in irradiated vessels at 8 wks after irradiation (panel a). The response of the rings to 10−7 M Ang II at different time points following exposure showed significant attenuation to vasoreactivity at 4, 8 and 52 wks after irradiation (* p<0.01 vs Ctrl, panel b).
Figure 7
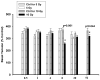
The effect of KCl on rat PA rings at different doses and time points. The blank and black columns represent 5 Gy and 10 Gy respectively. No significant differences were observed between 5 Gy and control rings (striped columns). Significant attenuation to KCl was noted at 8 wks and 52 wks following exposure to 10 Gy compared to controls (grey-striped columns). Each data is mean ± sem and expressed as % of baseline contraction (* p<0.05 vs Ctrl); _n_=16–40 rings from 4–11 rats.
Similar articles
- Vascular injury after whole thoracic x-ray irradiation in the rat.
Ghosh SN, Wu Q, Mäder M, Fish BL, Moulder JE, Jacobs ER, Medhora M, Molthen RC. Ghosh SN, et al. Int J Radiat Oncol Biol Phys. 2009 May 1;74(1):192-9. doi: 10.1016/j.ijrobp.2009.01.006. Int J Radiat Oncol Biol Phys. 2009. PMID: 19362237 Free PMC article. - Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis.
Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. Ghosh SN, et al. Int J Radiat Oncol Biol Phys. 2009 Dec 1;75(5):1528-36. doi: 10.1016/j.ijrobp.2009.07.1743. Int J Radiat Oncol Biol Phys. 2009. PMID: 19931735 Free PMC article. - Exacerbation of lung radiation injury by viral infection: the role of Clara cells and Clara cell secretory protein.
Manning CM, Johnston CJ, Hernady E, Miller JN, Reed CK, Lawrence BP, Williams JP, Finkelstein JN. Manning CM, et al. Radiat Res. 2013 Jun;179(6):617-29. doi: 10.1667/RR3279.1. Epub 2013 Apr 26. Radiat Res. 2013. PMID: 23621375 Free PMC article. - The delayed pulmonary syndrome following acute high-dose irradiation: a rhesus macaque model.
Garofalo M, Bennett A, Farese AM, Harper J, Ward A, Taylor-Howell C, Cui W, Gibbs A, Lasio G, Jackson W 3rd, MacVittie TJ. Garofalo M, et al. Health Phys. 2014 Jan;106(1):56-72. doi: 10.1097/HP.0b013e3182a32b3f. Health Phys. 2014. PMID: 24276550 - Vascular response to radiation injury in the rat lung.
Peterson LM, Evans ML, Graham MM, Eary JF, Dahlen DD. Peterson LM, et al. Radiat Res. 1992 Feb;129(2):139-48. Radiat Res. 1992. PMID: 1734443
Cited by
- Effect of angiotensin II on irradiation exacerbated decompression sickness.
Fan JF, Wang YK, Liu M, Liu GS, Min TJ, Chen RY, He Y. Fan JF, et al. Sci Rep. 2023 Jul 19;13(1):11659. doi: 10.1038/s41598-023-38752-z. Sci Rep. 2023. PMID: 37468556 Free PMC article. - Biomarkers to Predict Lethal Radiation Injury to the Rat Lung.
Medhora M, Gao F, Gasperetti T, Narayanan J, Himburg H, Jacobs ER, Clough AV, Fish BL, Szabo A. Medhora M, et al. Int J Mol Sci. 2023 Mar 15;24(6):5627. doi: 10.3390/ijms24065627. Int J Mol Sci. 2023. PMID: 36982722 Free PMC article. - Vascular regression in the kidney: changes in 3D vessel structure with time post-irradiation.
Mostaghimi S, Mehrvar S, Foomani FH, Narayanan J, Fish B, Camara AKS, Medhora M, Ranji M. Mostaghimi S, et al. Biomed Opt Express. 2022 Jul 26;13(8):4338-4352. doi: 10.1364/BOE.464426. eCollection 2022 Aug 1. Biomed Opt Express. 2022. PMID: 36032582 Free PMC article. - Age at Exposure to Radiation Determines Severity of Renal and Cardiac Disease in Rats.
Lenarczyk M, Kronenberg A, Mäder M, North PE, Komorowski R, Cheng Q, Little MP, Chiang IH, LaTessa C, Jardine J, Baker JE. Lenarczyk M, et al. Radiat Res. 2019 Jul;192(1):63-74. doi: 10.1667/RR15043.1. Epub 2019 May 16. Radiat Res. 2019. PMID: 31095446 Free PMC article. - Preclinical Pharmacokinetic and Pharmacodynamic Data To Support Cefoxitin Nebulization for the Treatment of Mycobacterium abscessus.
Mehta S, Aranzana-Climent V, Rammaert B, Grégoire N, Marchand S, Couet W, Buyck JM. Mehta S, et al. Antimicrob Agents Chemother. 2019 Jun 24;63(7):e02651-18. doi: 10.1128/AAC.02651-18. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31061149 Free PMC article.
References
- Arsalane K, Broeckaert F, Knoops B, Wiedig M, Toubeau G, Bernard A. Clara cell specific protein (CC16) expression after acute lung inflammation induced by intratracheal lipopolysaccharide administration. American Journal of Respiratory and Critical Care Medicine. 2000;161:1624–1630. - PubMed
- Baughmann RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207–213. - PubMed
- Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Annals of the New York Academy of Sciences. 2000;923:68–77. - PubMed
- Canzian M, Soeiro A, Taga MF, Farhat C, Barbas CSV, Capelozzi VL. Semiquantitative assessment of surgical lung biopsy: predictive value and impact on survival of patients with diffuse pulmonary infiltrate. Clinics. 2007;62:23–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI067734-010003/AI/NIAID NIH HHS/United States
- U19 AI067734-020003/AI/NIAID NIH HHS/United States
- U19 AI067734/AI/NIAID NIH HHS/United States
- U19 AI067734-030003/AI/NIAID NIH HHS/United States
- AI067734/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources