Oblimersen for the treatment of patients with chronic lymphocytic leukemia - PubMed (original) (raw)
Oblimersen for the treatment of patients with chronic lymphocytic leukemia
Bruce D Cheson. Ther Clin Risk Manag. 2007 Oct.
Abstract
Among adults in Western countries, chronic lymphocytic leukemia (CLL) is the most prevalent form of leukemia. CLL primarily affects the elderly and may be associated with multiple comorbidities. A cure has not been identified, and new treatment options are needed. Expression of Bcl-2 protein is associated with the pathogenesis of CLL and chemotherapy resistance. Oblimersen, a Bcl-2 antisense phosphorothioate oligonucleotide, is being evaluated in patients with CLL and other cancers; trials through Phase III have been completed. In the setting of relapsed/refractory CLL, single-agent oblimersen demonstrates modest activity, whereas the addition of oblimersen to fludarabine/cyclophosphamide significantly improves the rate of complete and nodular partial responses; moreover, these responses are durable and associated with clinical benefit. Oblimersen is more efficacious in relapsed rather than refractory patients. The side effect profile of oblimersen, alone or in combination with standard chemotherapy, is favorable compared with currently available chemotherapies. In the first cycle, an infusion reaction with or without tumor lysis syndrome is uncommon, and transient thrombocytopenia is observed. Catheter-related complications are associated with the need for continuous intravenous infusion of oblimersen over several days; other routes of administration are under clinical investigation. Oblimersen is a promising therapeutic approach for patients with relapsed CLL and should be further evaluated in the front-line setting.
Keywords: Bcl-2 antisense; G3139; Genasense®; chronic lymphocytic leukemia; oblimersen.
Figures
Figure 1
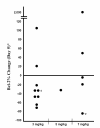
Bcl-2 response in peripheral blood mononuclear cells from pediatric patients with solid tumors by oblimersen dose: change from baseline (Day 0) on Day 5 (No Day 5 sample for 2 patients; response on Day 6 shown. Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from Rheingold SR, Hogarty MD, Blaney SM, et al. 2007. A Phase I trial of G3139, a bcl-2 antisense oligonucleotide, combined with doxorubicin and cyclophosphamide in children with relapsed solid tumors: A Children’s Oncology Group study. J Clin Oncol, 25 1512–18.
Figure 2
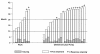
Overall response in previously untreated and previously treated patients with CLL: Phase II study.
Figure 3
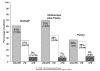
Duration of response (from date of first response) during and following protocol therapy among patients achieving a CR or nPR by treatment group: Phase III Study (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O'Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20).
Figure 4
Duration of response from date of best response by treatment group: Phase III study, intent-to-treat population (data on file, Genta Incorporated).
Figure 5
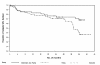
Survival with 3 years of follow-up by response and treatment group: Phase III study, intent-to-treat population (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20).
Figure 6
Kaplan-meier survival curves by treatment group: Phase III study, intent-to-treat population, fludarabine-responsive patients (Copyright © 2007. Reprinted with permission from the American Society of Clinical Oncology from O’Brien S, Moore JO, Boyd TE, et al. Randomized Phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol, 25:1114–20).
Similar articles
- Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia.
O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki A, Koziner B, Chanan-Khan AA, Seymour JF, Bociek RG, Pavletic S, Rai KR. O'Brien S, et al. J Clin Oncol. 2007 Mar 20;25(9):1114-20. doi: 10.1200/JCO.2006.07.1191. Epub 2007 Feb 12. J Clin Oncol. 2007. PMID: 17296974 Clinical Trial. - Potential therapeutic applications of oblimersen in CLL.
Koziner B. Koziner B. Oncology (Williston Park). 2004 Nov;18(13 Suppl 10):32-8. Oncology (Williston Park). 2004. PMID: 15651175 Review. - Oblimersen: Augmerosen, BCL-2 antisense oligonucleotide - Genta, G 3139, GC 3139, oblimersen sodium.
[No authors listed] [No authors listed] Drugs R D. 2007;8(5):321-34. doi: 10.2165/00126839-200708050-00006. Drugs R D. 2007. PMID: 17767397 Review. - Pharmacokinetic evaluation of oblimersen sodium for the treatment of chronic lymphocytic leukemia.
Advani PP, Paulus A, Masood A, Sher T, Chanan-Khan A. Advani PP, et al. Expert Opin Drug Metab Toxicol. 2011 Jun;7(6):765-74. doi: 10.1517/17425255.2011.579105. Epub 2011 Apr 27. Expert Opin Drug Metab Toxicol. 2011. PMID: 21521129 Review.
Cited by
- Chronic lymphocytic leukemia: interplay between noncoding RNAs and protein-coding genes.
Calin GA, Croce CM. Calin GA, et al. Blood. 2009 Nov 26;114(23):4761-70. doi: 10.1182/blood-2009-07-192740. Epub 2009 Sep 10. Blood. 2009. PMID: 19745066 Free PMC article. Review. - Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs.
Laikova KV, Oberemok VV, Krasnodubets AM, Gal'chinsky NV, Useinov RZ, Novikov IA, Temirova ZZ, Gorlov MV, Shved NA, Kumeiko VV, Makalish TP, Bessalova EY, Fomochkina II, Esin AS, Volkov ME, Kubyshkin AV. Laikova KV, et al. Molecules. 2019 Apr 17;24(8):1516. doi: 10.3390/molecules24081516. Molecules. 2019. PMID: 30999681 Free PMC article. Review. - Principles of microRNA involvement in human cancers.
Ling H, Zhang W, Calin GA. Ling H, et al. Chin J Cancer. 2011 Nov;30(11):739-48. doi: 10.5732/cjc.011.10243. Chin J Cancer. 2011. PMID: 22035854 Free PMC article. Review. - Transferrin receptor-targeted lipid nanoparticles for delivery of an antisense oligodeoxyribonucleotide against Bcl-2.
Yang X, Koh CG, Liu S, Pan X, Santhanam R, Yu B, Peng Y, Pang J, Golan S, Talmon Y, Jin Y, Muthusamy N, Byrd JC, Chan KK, Lee LJ, Marcucci G, Lee RJ. Yang X, et al. Mol Pharm. 2009 Jan-Feb;6(1):221-30. doi: 10.1021/mp800149s. Mol Pharm. 2009. PMID: 19183107 Free PMC article.
References
- Auer RL, Corbo M, Fegan CD, et al. Bcl-2 antisense (Genasense™) induces apoptosis and potentiates activity of both cytotoxic chemotherapy and rituximab in primary CLL cells. Blood; Proceedings of the 43rd Annual Meeting, American Society of Hematology; Dec 7–11; Orlando, FL, United States. 2001. p. 808a. abstract 3358.
- Aviram A, Rabizadeh E, Zimra Y, et al. Expression of bcl-2 and bax in cells isolated from B-chronic lymphocytic leukemia patients at different stages of the disease. Eur J Haematol. 2000;64:80–4. - PubMed
- Baird ME, Lucas DM, Mone AP, et al. G3139 promotes release of IL-8 by chronic lymphocytic leukemia cells cultured in vitro: potential implications for therapeutic use. Blood; Proceedings of the 43rd Annual Meeting, American Society of Hematology; 2001 Dec 7–11; Orlando, FL. 2001. p. 281b. abstract 4863.
- Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources